Acetylthiocholine Iodide
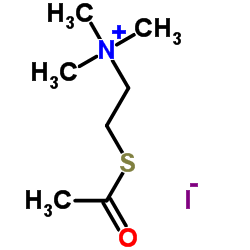
Acetylthiocholine Iodide structure
|
Common Name | Acetylthiocholine Iodide | ||
|---|---|---|---|---|
| CAS Number | 1866-15-5 | Molecular Weight | 289.177 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C7H16INOS | Melting Point | 205-210 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Neuroprotective activity and acetylcholinesterase inhibition of five Amaryllidaceae species: a comparative study.
Life Sci. 122 , 42-50, (2015) Amaryllidaceae alkaloids exhibit a wide range of physiological effects, of which the acetylcholinesterase (AChE) inhibitory activity is the most relevant. However, scientific evidence related to their neuroprotective effectiveness against glutamate-induced to... |
|
|
Chemical profile and biological activities of Veronica thymoides subsp. pseudocinerea.
Pharm. Biol. 53(3) , 334-9, (2015) In Turkey, Veronica species (Plantaginaceae) have been used as a diuretic and for wound healing in traditional medicine.To examine the fatty acid and essential oil profiles, the antioxidant, anticholinesterase, antimicrobial, and DNA damage effects of Veronic... |
|
|
Methamidophos alters sperm function and DNA at different stages of spermatogenesis in mice.
Toxicol. Appl. Pharmacol. 279(3) , 391-400, (2014) Methamidophos (MET) is a highly toxic organophosphate (OP) pesticide that is widely used in developing countries. MET has male reproductive effects, including decreased fertility. We evaluated MET effects on sperm quality, fertilization and DNA integrity, exp... |
|
|
Shogaol-huprine hybrids: dual antioxidant and anticholinesterase agents with β-amyloid and tau anti-aggregating properties.
Bioorg. Med. Chem. 22(19) , 5298-307, (2014) Multitarget compounds are increasingly being pursued for the effective treatment of complex diseases. Herein, we describe the design and synthesis of a novel class of shogaol-huprine hybrids, purported to hit several key targets involved in Alzheimer's diseas... |
|
|
Reconstituted mother tinctures of Gelsemium sempervirens L. improve memory and cognitive impairment in mice scopolamine-induced dementia model.
J. Ethnopharmacol. 159 , 274-84, (2014) Gelsemium sempervirens (L.) J.St.-Hil is a herb used for the treatment of various neuroses in both homeopathic and Ayurvedic systems. The present study examines whether Gelsemium reconstituted tincture can protect against scopolamine induced cognitive discrep... |
|
|
Chemical compositions and biological activities of the essential oils of Beilschmiedia madang Blume (Lauraceae).
Arch. Pharm. Res. 38(4) , 485-93, (2015) The present study aimed to examine the chemical compositions of the essential oils of Beilschmiedia madang and their antioxidant, antibacterial, antifungal, anticholinesterase and anti-tyrosinase activities. The major constituents of the essential oils of lea... |
|
|
Biological Activities and Chemical Composition of Methanolic Extracts of Selected Autochthonous Microalgae Strains from the Red Sea.
Mar. Drugs 13 , 3531-49, (2015) Four lipid-rich microalgal species from the Red Sea belonging to three different genera (Nannochloris, Picochlorum and Desmochloris), previously isolated as novel biodiesel feedstocks, were bioprospected for high-value, bioactive molecules. Methanol extracts ... |
|
|
Antioxidant and Anticholinesterase Activities of Essential Oils of Cinnamomum griffithii and C. macrocarpum.
Nat. Prod. Commun. 10 , 1465-8, (2015) The essential oils of Cinnamomum griffithii and C. macrocarpum were analyzed by GC and GC-MS and evaluated for their antioxidant and anticholinesterase activities. The essential oils of leaf and bark of C. grffithii were characterized by the presence of 30 co... |
|
|
Chemical Compositions and Biological Activities of Essential Oils of Beilschmiedia glabra.
Nat. Prod. Commun. 10 , 1297-300, (2015) This study was designed to examine the chemical compositions of essential oils from Beilschmiedia glabra and their antioxidant, antimicrobial, antityrosinase, acetylcholinesterase and anti-inflammatory activities. In total, 47 components were identified in th... |
|
|
In vitro and in vivo toxicological studies of V nerve agents: molecular and stereoselective aspects.
Toxicol. Lett. 232(2) , 438-48, (2015) In vitro inhibition data of cholinesterases (ChEs) and reactivation with HI 6 are presented for separated VX and VR enantiomers with high purity (enantiomer excess >99.999%). Inhibition rate constants for (-)-VR were fourfold higher than for (-)-VX. Marked hi... |