Thiomorpholine
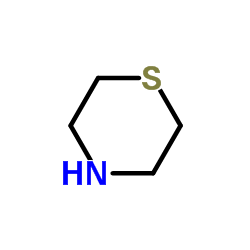
Thiomorpholine structure
|
Common Name | Thiomorpholine | ||
|---|---|---|---|---|
| CAS Number | 123-90-0 | Molecular Weight | 103.19 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 170.0±15.0 °C at 760 mmHg | |
| Molecular Formula | C4H9NS | Melting Point | 166-168 | |
| MSDS | Chinese USA | Flash Point | 60.0±0.0 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Synthesis and antitumor activities of some new N1-(flavon-6-yl)amidrazone derivatives.
Arch. Pharm. (Weinheim) 347(6) , 415-22, (2014) A new series of N1-(flavon-6-yl)amidrazones were synthesized by reacting the hydrazonoyl chloride derived from 6-aminoflavone with the appropriate sec-cyclic amines. The antitumor activities of these compounds were evaluated on breast cancer (MCF-7) and leuke... |
|
|
Structure-activity relationships of a novel pyranopyridine series of Gram-negative bacterial efflux pump inhibitors.
Bioorg. Med. Chem. 23(9) , 2024-34, (2015) Recently we described a novel pyranopyridine inhibitor (MBX2319) of RND-type efflux pumps of the Enterobacteriaceae. MBX2319 (3,3-dimethyl-5-cyano-8-morpholino-6-(phenethylthio)-3,4-dihydro-1H-pyrano[3,4-c]pyridine) is structurally distinct from other known G... |
|
|
Redox-neutral α-sulfenylation of secondary amines: ring-fused N,S-acetals.
Org. Lett. 16(13) , 3556-9, (2014) Secondary amines react with thiosalicylaldehydes in the presence of catalytic amounts of acetic acid to generate ring-fused N,S-acetals in redox-neutral fashion. A broad range of amines undergo α-sulfenylation, including challenging substrates such morpholine... |
|
|
A convenient route to optically pure α-alkyl-β-(sec-amino)alanines.
Amino Acids 42(6) , 2525-8, (2012) The cyclization of N-Boc-α-alkylserines to corresponding β-lactones under Mitsunobu reaction conditions and the ring opening with heterocyclic amines (pyrrolidine, piperidine, morpholine and thiomorpholine) produced N-Boc-α-alkyl-β-(sec-amino)alanines. The re... |
|
|
Synthesis, molecular docking and antitumor activity of novel pyrrolizines with potential as EGFR-TK inhibitors.
Bioorg. Chem. 59 , 124-9, (2015) A new series of pyrrolizine derivatives 4-8c were synthesized, their structures were confirmed by spectral and elemental analyses. Cytotoxic activity of these compounds was evaluated against breast (MCF7), colon (HCT116) and liver (HEPG2) cancer cell lines us... |
|
|
One-pot multicomponent synthesis of two novel thiolactone scaffolds.
Mol. Divers. 14(3) , 479-91, (2010) We designed two novel thiolactone scaffolds. Both scaffolds can be accessed by a convergent Ugi multicomponent reaction (MCR) and are, thus, amenable to library synthesis. Design, stereoselectivity, structures, full experimental details, and virtual libraries... |
|
|
Natural abundance nitrogen-15 nuclear magnetic resonance spectral studies on selected donors.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 58(12) , 2737-57, (2002) The natural abundance 15N-NMR chemical shifts of selected aliphatic amines, 2-substituted pyridine type compounds, bialicyclic tertiary amines have been measured as a function of the nature of the solvent. In the case of cyclic aliphatic amines, like piperidi... |
|
|
SnAP reagents for the transformation of aldehydes into substituted thiomorpholines--an alternative to cross-coupling with saturated heterocycles.
Angew. Chem. Int. Ed. Engl. 52(6) , 1705-8, (2013)
|
|
|
Synthesis of novel N-hydroxy heterocycles via intramolecular reductive cyclization of diketoximes by NaBH3CN.
Org. Biomol. Chem. 9 , 4642, (2011) A simple and efficient protocol for the construction of substituted piperazines, piperidines, thiomorpholines, decahydroquinolines, perhydrocyclopenta[b]pyridine, and pyrrolidines bearing N-hydroxy substituents through intramolecular reductive cyclization of ... |
|
|
Common degradative pathways of morpholine, thiomorpholine, and piperidine by Mycobacterium aurum MO1: evidence from (1)H-nuclear magnetic resonance and ionspray mass spectrometry performed directly on the incubation medium.
Appl. Environ. Microbiol. 66(8) , 3187-93, (2000) In order to see if the biodegradative pathways for morpholine and thiomorpholine during degradation by Mycobacterium aurum MO1 could be generalized to other heterocyclic compounds, the degradation of piperidine by this strain was investigated by performing (1... |