| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
acetic acid
CAS:64-19-7 |
|
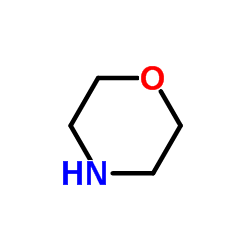 |
morpholine
CAS:110-91-8 |
|
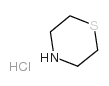 |
Thiomorpholine Hydrochloride
CAS:5967-90-8 |
|
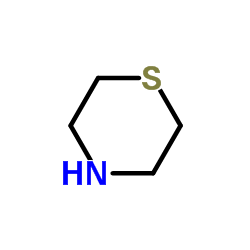 |
Thiomorpholine
CAS:123-90-0 |