Disulfur dichloride
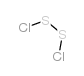
Disulfur dichloride structure
|
Common Name | Disulfur dichloride | ||
|---|---|---|---|---|
| CAS Number | 10025-67-9 | Molecular Weight | 135.03600 | |
| Density | 1.688 g/mL at 25 °C(lit.) | Boiling Point | 138 °C(lit.) | |
| Molecular Formula | Cl2S2 | Melting Point | −80 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 118ºC | |
| Symbol |



GHS05, GHS06, GHS09 |
Signal Word | Danger | |
|
Synthesis of taurine analogues from alkenes: preparation of 2-aminocycloalkanesulfonic acids.
Adv. Exp. Med. Biol. 483 , 399-401, (2000) (+/-)trans 2-Aminocyclohexanesulfonic acid and (+/-)trans 2-aminocyclopentanesulfonic acid were prepared from cyclohexene and cyclopentene respectively by sulfur monochloride addition, followed by oxidation to 2-chlorosulfonic acid and substitution of chlorin... |
|
|
[Eye burn caused by sulfur monochloride].
Bull. Soc. Ophtalmol. Fr. 87(3) , 429-30, (1987)
|
|
|
Hypochlorite-induced oxidation of thiols: formation of thiyl radicals and the role of sulfenyl chlorides as intermediates.
Free Radic. Res. 33(6) , 719-29, (2000) Activated phagocytic cells generate hypochlorite (HOCl) via release of hydrogen peroxide and the enzyme myeloperoxidase. HOCl plays an important role in bacterial cell killing, but excessive or misplaced production of HOCI is also known to cause tissue damage... |
|
|
Ab initio calculations on SCl2 and low-lying cationic states of SCl2+: Franck-Condon simulation of the UV photoelectron spectrum of SCl2.
J. Chem. Phys. 125(10) , 104303, (2006) Geometry optimization calculations were carried out on the (approximate)X (1)A(1) state of SCl(2) and the (approximate)X(2)B(1), (approximate)A(2)B(2), (approximate)B(2)A(1), (approximate)C(2)A(1), (approximate)D(2)A(2), and (approximate)E (2)B(2) states of S... |