Spiramycin
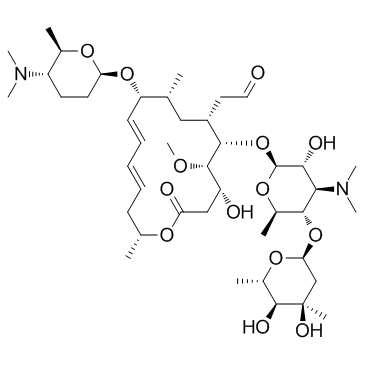
Spiramycin structure
|
Common Name | Spiramycin | ||
|---|---|---|---|---|
| CAS Number | 8025-81-8 | Molecular Weight | 843.053 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 913.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C43H74N2O14 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 506.4±34.3 °C | |
|
Regulation of the biosynthesis of the macrolide antibiotic spiramycin in Streptomyces ambofaciens.
J. Bacteriol. 192(21) , 5813-21, (2010) Streptomyces ambofaciens synthesizes the macrolide antibiotic spiramycin. The biosynthetic gene cluster for spiramycin has been characterized for S. ambofaciens. In addition to the regulatory gene srmR (srm22), previously identified (M. Geistlich et al., Mol.... |
|
|
A spicamycin derivative (KRN5500) provides neuropathic pain relief in patients with advanced cancer: a placebo-controlled, proof-of-concept trial.
J. Pain Symptom Manage. 43(4) , 679-93, (2012) Neuropathic pain in patients with cancer can be difficult to treat effectively.The purpose of the study was to determine safety and efficacy of KRN5500, a novel, spicamycin-derived, nonopioid analgesic agent, in patients with advanced cancer and neuropathic p... |
|
|
Influences of two antibiotic contaminants on the production, release and toxicity of microcystins
Ecotoxicol. Environ. Saf. 77 , 79-87, (2012) The influences of spiramycin and amoxicillin on the algal growth, production and release of target microcystins (MCs), MC-LR, MC-RR and MC-YR, in Microcystis aeruginosa were investigated through the seven-day exposure test. Spiramycin were more toxic to M. ae... |
|
|
Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis.
Antimicrob. Agents Chemother. 56(4) , 1762-8, (2012) Toxoplasma gondii is a parasite that generates latent cysts in the brain; reactivation of these cysts may lead to fatal toxoplasmic encephalitis, for which treatment remains unsuccessful. We assessed spiramycin pharmacokinetics coadministered with metronidazo... |
|
|
Antioxidant responses and degradation of two antibiotic contaminants in Microcystis aeruginosa.
Ecotoxicol. Environ. Saf. 86 , 23-30, (2012) Cyanobacteria may interact with antibiotic contaminants in aquatic environments, but the interaction effects and mechanisms remain unclear. In the present study, aqueous culture of Microcystis aeruginosa was exposed to 50ng/l-1μg/l of spiramycin and amoxicill... |
|
|
Pharmacodynamics and pharmacokinetics of spiramycin and their clinical significance.
Clin. Pharmacokinet. 34(4) , 303-10, (1998) The absolute bioavailability of oral spiramycin is generally within the range of 30 to 40%. After a 1 g oral dose, the maximum serum drug concentration was found to be within the range 0.4 to 1.4 mg/L. The tissue distribution of spiramycin is extensive. The v... |
|
|
Cryptosporidiosis: a rare and severe infection in a pediatric renal transplant recipient.
Pediatr. Transplant. 16(4) , E115-9, (2012) Cryptosporidium is an intracellular protozoan parasite that causes gastroenteritis in human. In immunocompromised individuals, cryptosporidium causes far more serious disease. There is no effective specific therapy for cryptosporidiosis, and spontaneous recov... |
|
|
Azithromycin and spiramycin induce anti-inflammatory response in human trophoblastic (BeWo) cells infected by Toxoplasma gondii but are able to control infection
Placenta 32(11) , 838-44, (2011) Toxoplasma gondii is an important pathogen which may cause fetal infection if primary infection. Our previous studies have used human choriocarcinoma trophoblastic cells (BeWo cell line) as experimental model of T. gondii infection involving placental microen... |
|
|
[Prevention of mother-to-child transmission of toxoplasmosis: perspectives].
Gynecol. Obstet. Fertil. 40(10) , 591-8, (2012) In France, screening for toxoplasmosis is mandatory during pregnancy, whereas it is not performed in most other countries. The rationale for prenatal screening is to allow for several levels of intervention: primary prevention by health education; in case of ... |
|
|
Kinetic study of irreversible inhibition of an enzyme consumed in the reaction it catalyses. Application to the inhibition of the puromycin reaction by spiramycin and hydroxylamine.
J. Enzym. Inhib. 12(2) , 79-99, (1997) A systematic procedure for the kinetic study of irreversible inhibition when the enzyme is consumed in the reaction which it catalyses, has been developed and analysed. Whereas in most reactions the enzymes are regenerated after each catalytic event and serve... |