Sodium carboxyl methylstarch
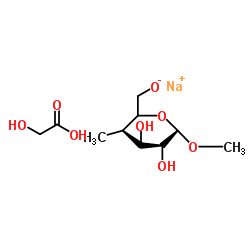
Sodium carboxyl methylstarch structure
|
Common Name | Sodium carboxyl methylstarch | ||
|---|---|---|---|---|
| CAS Number | 9063-38-1 | Molecular Weight | 290.243 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C10H19NaO8 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
|
II. Technological approaches to improve the dissolution behavior of nateglinide, a lipophilic insoluble drug: co-milling.
Int. J. Pharm. 454(1) , 568-72, (2013) Nateglinide is an oral antidiabetic agent that should be administered 10-30 min before the meal, but it shows low and pH-dependent solubility that may reduce its oral bioavailability. To improve nateglinide dissolution rate, the active was co-milled with thre... |
|
|
Ultrasonic approach for viscoelastic and microstructure characterization of granular pharmaceutical tablets.
Int. J. Pharm. 454(1) , 333-43, (2013) The mechanical properties of a solid dosage, defined by its granular micro-structure and geometry, play a key role in its dissolution profile and performance. An ultrasonic method for extracting the viscoelastic material properties and granular structure of d... |
|
|
Preparation of cross-linked carboxymethyl jackfruit starch and evaluation as a tablet disintegrant.
Pak. J. Pharm. Sci. 24(4) , 415-20, (2011) The main purposes of this study are to prepare cross-linked carboxymethyl jackfruit starch (CL-CMJF) and to evaluate its pharmaceutical property as a tablet disintegrant. CL-CMJF was prepared by a dual carboxymethyl-crosslinking reaction in a flask containing... |
|
|
Dissolution rate enhancement of gliclazide by ordered mixing.
Acta. Pharm. 61(3) , 323-34, (2011) The poorly water soluble antidiabetic drug gliclazide was selected to study the effect of excipients on dissolution rate enhancement. Ordered mixtures of micronized gliclazide with lactose, mannitol, sorbitol, maltitol and sodium chloride were prepared by man... |
|
|
Development and optimization of dextromethorphan hydrobromide oral disintegrating tablets: effect of formulation and process variables.
Pharm. Dev. Technol. 18(2) , 454-63, (2013) Orally disintegrating tablets (ODTs), which disintegrate rapidly (<1 min) in the mouth and do not require water for administration, have become a very popular dosage form. The study aims to develop a simple and inexpensive method of manufacturing ODTs of a sp... |
|
|
[Comparative evaluation of the anionic superdisintegrant incorporation mode on the quality of ranitidine tablets].
Rev. Med. Chir. Soc. Med. Nat. Iasi. 116(1) , 336-40, (2012) The present study was based on the impact of the superdisintegrants incorporation mechanism on the immediate realese of the tablets final performances. The aim was the selection of the working method to obtain Ranitidine 150 mg tablets with the desiderate qua... |
|
|
A comparison of chitosan-silica and sodium starch glycolate as disintegrants for spheronized extruded microcrystalline cellulose pellets.
Drug Dev. Ind. Pharm. 37(7) , 825-31, (2011) Chitosan-silica coprecipitate (C-S) has recently been proposed as a tablet disintegrant. In this study we compared it with a 1:1 physical mixture of chitosan and silica (C/S) at the same composition as the coprecipitate, and with the widely used commercial di... |
|
|
Design, development and optimization of a novel time and pH-dependent colon targeted drug delivery system.
Pharm. Dev. Technol. 14(1) , 62-9, (2009) The aim of present study was to develop a time- and pH-dependent system for delivering mesalamine to the colon. The system consists of the core tablet of mesalamine which is compression coated with hydroxypropyl methylcellulose (HPMC K4M) (time-dependent fact... |
|
|
Functional assessment of four types of disintegrants and their effect on the spironolactone release properties.
AAPS PharmSciTech 13(4) , 1054-62, (2012) Spironolactone is a drug derived from sterols that exhibits an incomplete oral absorption due to its low water solubility and slow dissolution rate. In this study, formulations of spironolactone with four disintegrants named as croscarmellose sodium, crospovi... |
|
|
Formulation development and in vitro evaluation of solidified self-microemulsion in the form of tablet containing atorvastatin calcium.
Drug Dev. Ind. Pharm. 39(11) , 1742-9, (2013) The objective of our present study was to prepare solid self-microemulsion in the form of tablet of a poorly water soluble drug, Atorvastatin calcium (ATNC) to increase the solubility, dissolution rate, and minimize the hazards experienced from liquid emulsio... |