Indole-5-carboxaldehyde
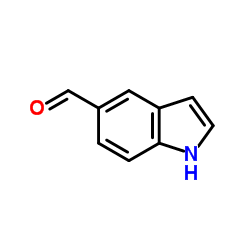
Indole-5-carboxaldehyde structure
|
Common Name | Indole-5-carboxaldehyde | ||
|---|---|---|---|---|
| CAS Number | 1196-69-6 | Molecular Weight | 145.158 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 339.1±15.0 °C at 760 mmHg | |
| Molecular Formula | C9H7NO | Melting Point | 100-103 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 166.8±27.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis and antifungal activity of novel streptochlorin analogues.
Eur. J. Med. Chem. 92 , 776-83, (2015) Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of pimprinine... |
|
|
Synthesis and antiproliferative activity of novel 2-aryl-4-benzoyl-imidazole derivatives targeting tubulin polymerization.
Bioorg. Med. Chem. 19 , 4782-95, (2011) We previously reported the discovery of 2-aryl-4-benzoyl-imidazoles (ABI-I) as potent antiproliferative agents for melanoma. To further understand the structural requirements for the potency of ABI analogs, gain insight in the structure-activity relationships... |
|
|
Synthesis and biological evaluation of achiral indole-substituted titanocene dichloride derivatives.
Int J Med Chem 2012 , 905981, (2015) Six new titanocene compounds have been isolated and characterised. These compounds were synthesised from their fulvene precursors using Super Hydride (LiBEt3H) followed by transmetallation with titanium tetrachloride to yield the corresponding titanocene dich... |
|
|
Synthesis and structure-affinity relationships of novel dibenzylideneacetone derivatives as probes for β-amyloid plaques.
J. Med. Chem. 54 , 2225, (2011) A new and extensive set of dibenzylideneacetone derivatives was synthesized and screened for affinity toward Aβ(1-42) aggregates. Structure-activity relationships revealed the binding of dibenzylideneacetones to be affected by various substituents. The introd... |
|
|
Amyloid-β probes: Review of structure-activity and brain-kinetics relationships.
Beilstein J. Org. Chem. 9 , 1012-44, (2013) The number of people suffering from Alzheimer's disease (AD) is expected to increase dramatically in the coming years, placing a huge burden on society. Current treatments for AD leave much to be desired, and numerous research efforts around the globe are foc... |
|
|
Enhancing the Pharmacokinetic Properties of Botulinum Neurotoxin Serotype A Protease Inhibitors Through Rational Design.
ACS Chem. Neurosci. 2 , 288, (2011) Botulinum neurotoxin (BoNT), the etiological agent that causes the neuroparalytic disease botulism, has become a highly studied drug target in light of the potential abuse of this toxin as a weapon of bioterrorism. In particular, small molecule inhibitors of ... |
|
|
Curcumin analogues as possible anti-proliferative & anti-inflammatory agents.
Eur. J. Med. Chem. 46 , 2722, (2011) A series of novel curcumin analogues has been designed, synthesized and tested in vitro/in vivo as potential multi-target agents. Their anti-proliferative and anti-inflammatory activities were studied. Compounds 1b and 2b were stronger inhibitors of soybean l... |
|
|
Structure-based drug design of novel Aurora kinase A inhibitors: structural basis for potency and specificity.
J. Med. Chem. 52 , 1050, (2009) Aurora kinases have emerged as attractive targets for the design of anticancer drugs. Through structure-based virtual screening, novel pyrazole hit 8a was identified as Aurora kinase A inhibitor (IC(50) = 15.1 microM). X-ray cocrystal structure of 8a in compl... |
|
|
Heterocyclic bibenzimidazole derivatives as topoisomerase I inhibitors.
Bioorg. Med. Chem. Lett. 10 , 719, (2000) A series of 2'-heterocyclic derivatives of 5-phenyl-2,5'-1H-bibenzimidazoles were evaluated for topoisomerase I poisoning activity and cytotoxicity. Topo I poisoning activity was associated with 2'-derivatives that possessed a hydrogen atom capable of hydroge... |
|
|
Catalytic asymmetric synthesis of protected tryptophan regioisomers.
J. Org. Chem. 67 , 6256, (2002) Tryptophan 1 (Trp) is superior to all other naturally occurring peptide residues in its ability to bind cations (the cation-pi interaction). In an effort to expand the toolbox of Trp-like amino acids, in this note we report catalytic asymmetric syntheses of T... |