Structure-based drug design of novel Aurora kinase A inhibitors: structural basis for potency and specificity.
Mohane Selvaraj Coumar, Jiun-Shyang Leou, Paritosh Shukla, Jian-Sung Wu, Ajay Kumar Dixit, Wen-Hsing Lin, Chun-Yu Chang, Tzu-Wen Lien, Uan-Kang Tan, Chun-Hwa Chen, John T-A Hsu, Yu-Sheng Chao, Su-Ying Wu, Hsing-Pang Hsieh
Index: J. Med. Chem. 52 , 1050, (2009)
Full Text: HTML
Abstract
Aurora kinases have emerged as attractive targets for the design of anticancer drugs. Through structure-based virtual screening, novel pyrazole hit 8a was identified as Aurora kinase A inhibitor (IC(50) = 15.1 microM). X-ray cocrystal structure of 8a in complex with Aurora A protein revealed the C-4 position ethyl carboxylate side chain as a possible modification site for improving the potency. On the basis of this insight, bioisosteric replacement of the ester with amide linkage and changing the ethyl substituent to hydrophobic 3-acetamidophenyl ring led to the identification of 12w with a approximately 450-fold improved Aurora kinase A inhibition potency (IC(50) = 33 nM), compared to 8a. Compound 12w showed selective inhibition of Aurora A kinase over Aurora B/C, which might be due to the presence of a unique H-bond interaction between the 3-acetamido group and the Aurora A nonconserved Thr217 residue, which in Aurora B/C is Glu and found to sterically clash with the 3-acetamido group in modeling studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
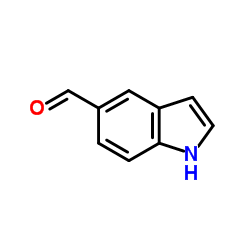 |
Indole-5-carboxaldehyde
CAS:1196-69-6 |
C9H7NO |
|
Synthesis and antifungal activity of novel streptochlorin an...
2015-03-06 [Eur. J. Med. Chem. 92 , 776-83, (2015)] |
|
Synthesis and antiproliferative activity of novel 2-aryl-4-b...
2011-08-15 [Bioorg. Med. Chem. 19 , 4782-95, (2011)] |
|
Synthesis and biological evaluation of achiral indole-substi...
2012-01-01 [Int J Med Chem 2012 , 905981, (2015)] |
|
Synthesis and structure-affinity relationships of novel dibe...
2011-04-14 [J. Med. Chem. 54 , 2225, (2011)] |
|
Amyloid-β probes: Review of structure-activity and brain-kin...
2013-01-01 [Beilstein J. Org. Chem. 9 , 1012-44, (2013)] |