8-Azaadenine
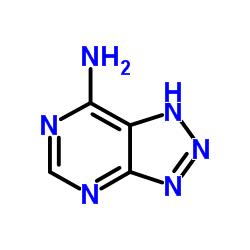
8-Azaadenine structure
|
Common Name | 8-Azaadenine | ||
|---|---|---|---|---|
| CAS Number | 1123-54-2 | Molecular Weight | 136.115 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 563.9±30.0 °C at 760 mmHg | |
| Molecular Formula | C4H4N6 | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 329.4±11.8 °C | |
|
Intracellular generation of ROS by 3,5-dimethylaminophenol: persistence, cellular response, and impact of molecular toxicity.
Toxicol. Sci. 141(1) , 300-13, (2014) Epidemiological studies have demonstrated extensive human exposure to the monocyclic aromatic amines, particularly to 3,5-dimethylaniline, and found an association between exposure to these compounds and risk for bladder cancer. Little is known about molecula... |
|
|
Intersystem crossing to excited triplet state of aza analogues of nucleic acid bases in acetonitrile.
J. Phys. Chem. A 113(44) , 12088-93, (2009) Excited state characteristics of aza analogues of nucleic acid bases, 8-azaadenine (8AA), 5-azacytosine (5AC), 8-azaguanine (8AG), and 6-azauracil (6AU), in acetonitrile solution were comprehensively investigated with steady state absorption and emission spec... |
|
|
[Effects of 5-bromouracil and 5-bromodeoxyuridine in combination with 8-aza-adenine on the UV sensitivity of bacteria].
Z. Allg. Mikrobiol. 23(8) , 495-501, (1983) The presence of 5-bromouracil (BU) as well as 5-bromo-2-deoxyuridine (BUdR) in the cultivation media of bacteria results in the distinct increase of UV sensitivity. With the nucleic acid base analogue 8-azaadenine (8-AA) a similar effect was confirmed, howeve... |
|
|
N6-1,3-diphenylurea derivatives of 2-phenyl-9-benzyladenines and 8-azaadenines: synthesis and biological evaluation as allosteric modulators of A2A adenosine receptors.
Eur. J. Med. Chem. 43 , 1639-47, (2008) Some 1-[4-(9-benzyl-2-phenyl-9H-purin-6-ylamino)-phenyl]-3-phenyl-urea derivatives and some 1-[4-(9-benzyl-2-phenyl-9H-8-azapurin-6-ylamino)-phenyl]-3-phenyl-urea derivatives were synthesised and evaluated for their interaction with adenosine receptors. It wa... |
|
|
N6-cycloalkyl-2-phenyl-3-deaza-8-azaadenines: a new class of A1 adenosine receptor ligands. A comparison with the corresponding adenines and 8-azaadenines.
Eur. J. Med. Chem. 38(11-12) , 983-90, (2003) Several 9-benzyl-N6-cycloalkyl-2-phenyladenines, 9-benzyl-N6-cycloalkyl-2-phenyl-8-azaadenines and 4-cycloalkylamino-1-benzyl-6-phenyl-1H-1,2,3-triazolo[4,5-c]pyridines were prepared and assayed as A1 adenosine receptor ligands. The 1H-1,2,3-triazolo[4,5-c]py... |
|
|
New 2-(2'-phenyl-9'-benzyl-8'-azapurin-6'-ylamino)-carboxylic acid methylesters as ligands for A1 adenosine receptors.
Il Farmaco 56(12) , 929-31, (2001) Synthesis of a series of new 2-phenyl-9-benzyl-8-azaadenines bearing on N6 an alkyl or aralkyl chain having a carbonyloxymethyl group on the carbon bound to N6 were reported. The ester group could assure to the molecule a better water-solubility than the 8-az... |
|
|
High rate of multilocus deletion in a human tumor cell line.
Hum. Mol. Genet. 2(2) , 165-71, (1993) The nature of recessive mutations at the autosomal locus encoding the purine salvage enzyme adenine phosphoribosyl transferase (APRT) was analyzed in a highly malignant human tumor cell line (the colorectal carcinoma line SW620). Mutant strains resistant to t... |
|
|
Synthesis, antiviral and cytostatic activities of carbocyclic nucleosides incorporating a modified cyclopentane ring. Part 2: Adenosine and uridine analogues.
Nucleosides Nucleotides 17(7) , 1255-66, (1998) Six new carbocyclic nucleosides were prepared by mounting a purine (compounds 5-7), 8-azapurine (compounds 9 and 10) or pyrimidine (compound 13) base on the amino group of (1R,cis)-3-(aminomethyl)-1,2,2-trimethylcyclopentylmethanol (2). The antiviral activity... |
|
|
AtAzg1 and AtAzg2 comprise a novel family of purine transporters in Arabidopsis
FEBS Lett. 583(2) , 481-6, (2009) In plants, nucleobase biochemistry is highly compartmented relying upon a well-regulated and selective membrane transport system. In Arabidopsis two proteins, AtAzg1 and AtAzg2, show substantial amino acid sequence similarity to the adenine–guanine–hypoxanthi... |
|
|
Endogenous cytokinins in Cocos nucifera L. in vitro cultures obtained from plumular explants.
Plant Cell Rep. 29(11) , 1227-34, (2010) Auxin induces in vitro somatic embryogenesis in coconut plumular explants through callus formation. Embryogenic calli and non-embryogenic calli can be formed from the initial calli. Analysis of endogenous cytokinins showed the occurrence of cytokinins with ar... |