| Structure | Name/CAS No. | Articles |
|---|---|---|
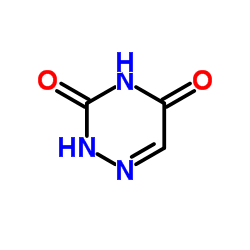 |
6-Azauracil
CAS:461-89-2 |
|
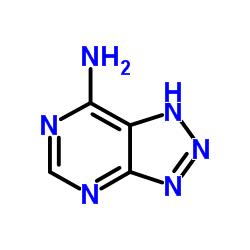 |
8-Azaadenine
CAS:1123-54-2 |
|
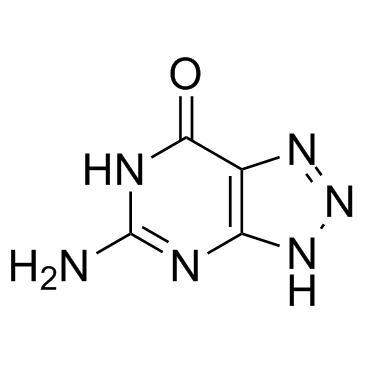 |
8-Azaguanine
CAS:134-58-7 |