Nucleosides & nucleotides
1998-07-01
Synthesis, antiviral and cytostatic activities of carbocyclic nucleosides incorporating a modified cyclopentane ring. Part 2: Adenosine and uridine analogues.
M I Nieto, J M Blanco, O Caamaño, F Fernández, X García-Mera, J Balzarini, E Padalko, J Neyts, E De Clercq
Index: Nucleosides Nucleotides 17(7) , 1255-66, (1998)
Full Text: HTML
Abstract
Six new carbocyclic nucleosides were prepared by mounting a purine (compounds 5-7), 8-azapurine (compounds 9 and 10) or pyrimidine (compound 13) base on the amino group of (1R,cis)-3-(aminomethyl)-1,2,2-trimethylcyclopentylmethanol (2). The antiviral activity of compounds 5-7, 10 and 13, and their cytostatic activity, were evaluated. At subtoxic concentrations, the compounds showed no or marginal antiviral activity. Compound 5 showed moderate inhibition on tumor cell proliferation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
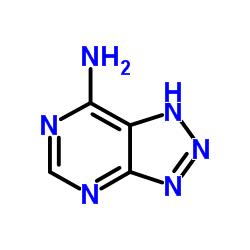 |
8-Azaadenine
CAS:1123-54-2 |
C4H4N6 |
Related Articles:
More...
|
Intracellular generation of ROS by 3,5-dimethylaminophenol: ...
2014-09-01 [Toxicol. Sci. 141(1) , 300-13, (2014)] |
|
Intersystem crossing to excited triplet state of aza analogu...
2009-11-05 [J. Phys. Chem. A 113(44) , 12088-93, (2009)] |
|
[Effects of 5-bromouracil and 5-bromodeoxyuridine in combina...
1983-01-01 [Z. Allg. Mikrobiol. 23(8) , 495-501, (1983)] |
|
N6-1,3-diphenylurea derivatives of 2-phenyl-9-benzyladenines...
2008-08-01 [Eur. J. Med. Chem. 43 , 1639-47, (2008)] |
|
N6-cycloalkyl-2-phenyl-3-deaza-8-azaadenines: a new class of...
2003-01-01 [Eur. J. Med. Chem. 38(11-12) , 983-90, (2003)] |