Diethyl sulfide
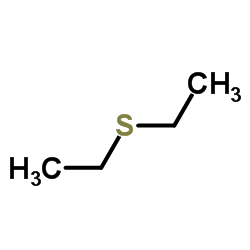
Diethyl sulfide structure
|
Common Name | Diethyl sulfide | ||
|---|---|---|---|---|
| CAS Number | 352-93-2 | Molecular Weight | 90.187 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 94.4±8.0 °C at 760 mmHg | |
| Molecular Formula | C4H10S | Melting Point | −100 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | -9.4±0.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
|
QSPR modeling of octanol/water partition coefficient for vitamins by optimal descriptors calculated with SMILES.
Eur. J. Med. Chem. 43 , 714-40, (2008) Simplified molecular input line entry system (SMILES) has been utilized in constructing quantitative structure-property relationships (QSPR) for octanol/water partition coefficient of vitamins and organic compounds of different classes by optimal descriptors.... |
|
|
Et2SBrSbCl5Br: an effective reagent for direct bromonium-induced polyene cyclizations.
Angew. Chem. Int. Ed. Engl. 48 , 7899-7903, (2009)
|
|
|
Quantification of volatile sulfur compounds in complex gaseous matrices by solid-phase microextraction.
J. Chromatogr. A. 963(1-2) , 57-64, (2002) Procedures were assessed for quantifying nine volatile sulfur compounds found in complex gaseous samples collected at a biogas-production plant and a sewage treatment plant. The target compounds were extracted by solid-phase microextraction (using the 75-micr... |
|
|
Detection and characterization of two major ethylated deoxyguanosine adducts by high performance liquid chromatography, electrospray mass spectrometry, and 32P-postlabeling. Development of an approach for detection of phosphotriesters.
Chem. Res. Toxicol. 10(1) , 70-7, (1997) Postlabeling can be one of the most sensitive methods for the measurement of DNA adducts. However, for the determination of alkylated adducts, essential requirements are standards which must be fully chemically characterized. In order to develop a postlabelin... |
|
|
Synthesis and stable isotope dilution assay of ethanethiol and diethyl disulfide in wine using solid phase microextraction. Effect of aging on their levels in wine.
J. Agric. Food Chem. 50(23) , 6653-8, (2002) Ethanethiol and diethyl disulfide (DEDS) most often occurred at levels above their olfactive threshold in wines with nauseous sulfur-linked smells. As ethanethiol is very oxidizable and chemically reactive, a stable isotopic dilution analysis of both ethaneth... |
|
|
Characteristics of sulfur response in a micro-flame photometric detector.
J. Chromatogr. A. 1105(1-2) , 66-70, (2006) A recently reported micro-flame photometric detector (microFPD) has been examined in greater detail for its sulfur response characteristics. While supporting an "upside down" flame on a stainless steel capillary burner (delivering oxygen) in a counter flowing... |
|
|
Experimental and theoretical studies of the reaction of the OH radical with alkyl sulfides: 2. Kinetics and mechanism of the OH initiated oxidation of methylethyl and diethyl sulfides; observations of a two channel oxidation mechanism.
Phys. Chem. Chem. Phys. 9(31) , 4370-82, (2007) A pulsed laser photolysis-pulsed laser induced fluorescence technique has been employed to measure rate coefficients for the OH initiated oxidation of methylethyl sulfide (MES) and diethylsulfide (DES). In the absence of oxygen and at low sulfide concentratio... |
|
|
Breakthrough behavior of diethyl sulphide vapor on active carbon systems.
J. Hazard. Mater. 139(1) , 38-43, (2007) Breakthrough behavior of diethyl sulphide vapors on carbon systems such as active carbon, NaOH/CrO3/C, NaOH/CrO3/EDA/C and RuCl3/C has been studied by using modified Wheeler equation and the same was used to calculate the pseudo-first-order rate constant (kv)... |
|
|
ATP and GTP are essential for olfactory response.
Neurosci. Lett. 73(3) , 253-8, (1987) The steady-state conductance of planar bimolecular lipid membranes (BLMs) modified with rat olfactory epithelial homogenate (ROH) becomes sensitive to very low concentrations of odorant in the presence of adenosine triphosphate (ATP) and guanosine triphosphat... |
|
|
Femtochemistry uncovers the nature of electron transfer reactions.
Proc. Natl. Acad. Sci. U. S. A. 96(8) , 4219-20, (1999)
|