(R)-(+)-1-Phenylethylamine
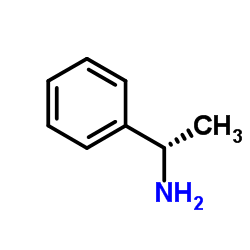
(R)-(+)-1-Phenylethylamine structure
|
Common Name | (R)-(+)-1-Phenylethylamine | ||
|---|---|---|---|---|
| CAS Number | 3886-69-9 | Molecular Weight | 121.180 | |
| Density | 0.95 | Boiling Point | 184-186 ºC | |
| Molecular Formula | C8H11N | Melting Point | -10 °C | |
| MSDS | Chinese USA | Flash Point | 75 ºC | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Interactions of an asymmetric amine with a non-C2 symmetric Cu-salen complex: an EPR/ENDOR and HYSCORE investigation.
Phys. Chem. Chem. Phys. 13(45) , 20427-34, (2011) Single enantiomers of R-/S-methylbenzylamine (MBA) were found to selectively form adducts with the chiral non-C(2) symmetric Cu-salen complex N-(3,5-di-tert-butylsalicylidene)-N'-(salicylidene)-cyclohexane-1,2-diamine copper(II), hereafter labelled [Cu(3)]. T... |
|
|
Synthesis, Crystal Structure, Absolute Configuration and Antitumor Activity of the Enantiomers of 5-Bromo-2-chloro-N-(1-phenylethyl)pyridine-3-sulfonamide.
Molecules 20 , 20926-38, (2015) Pyridinesulfonamide is an important fragment which has a wide range of applications in novel drugs. R- and S-isomers of 5-bromo-2-chloro-N-(1-phenylethyl)pyridine-3-sulfonamide have been synthesized, and the stereostructures have been researched. Single cryst... |
|
|
Towards the chiral metabolomics: Liquid chromatography-mass spectrometry based DL-amino acid analysis after labeling with a new chiral reagent, (S)-2,5-dioxopyrrolidin-1-yl-1-(4,6-dimethoxy-1,3,5-triazin-2-yl)pyrrolidine-2-carboxylate, and the application to saliva of healthy volunteers.
Anal. Chim. Acta 875 , 73-82, (2015) A novel triazine-type chiral derivatization reagent, i.e., (S)-2,5-dioxopyrrolidin-1-yl-1-(4,6-dimethoxy-1,3,5-triazin-2-yl) pyrrolidine-2-carboxylate (DMT-(S)-Pro-OSu), was developed for the highly sensitive and selective detection of chiral amines and amino... |
|
|
Enantioselective nanofiber-spinning of chiral calixarene receptor with guest.
Chem. Commun. (Camb.) (32) , 3398-400, (2007) Chiral para-tert-butylcalix[4]arene bearing (S)-alpha-methylbenzylamine groups at lower rim only self-assembles with one of two enantiomers of 2,3-dibenzoyltartaric acid into coiled nanofibers and the coiled nanofibers only stack with the nanofibers having th... |
|
|
Characterization of free and immobilized (S)-aminotransferase for acetophenone production.
Appl. Microbiol. Biotechnol. 76(4) , 843-51, (2007) Enzyme immobilization often improves process economics, but changes in kinetic properties may also occur. The immobilization of a recombinant thermostable (S)-aminotransferase was made by entrapment on calcium alginate-3% (w/v)-and tested with (S)-(-)-(alpha)... |
|
|
Synthesis and evaluation of atropos dihydro-5H-dibenzazepinium halide PTCs derived from α-methylbenzylamine.
Org. Biomol. Chem. 10(25) , 4968-76, (2012) A short synthetic route to diastereoisomeric atropos dihydro-5H-dibenz[c,e]azepinium salts via reaction of a single enantiomer of (R)-α-methylbenzylamine with a racemic atropos biphenol derivative is described. Compounds prepared via this approach are used to... |
|
|
Chiral self-discrimination of the enantiomers of alpha-phenylethylamine derivatives in proton NMR.
Magn. Reson. Chem. 47(5) , 423-7, (2009) Two types of chiral analytes, the urea and amide derivatives of alpha-phenylethylamine, were prepared. The effect of inter-molecular hydrogen-bonding interaction on self-discrimination of the enantiomers of analytes has been investigated using high-resolution... |
|
|
LC-MS identification of derivatized free fatty acids from adipocere in soil samples.
J. Sep. Sci. 33(2) , 143-54, (2010) Free fatty acids were derivatized as amides (DFFA) by reaction with (R)-(+)-1-phenylethylamine, using a simple, fast and robust reaction scheme. A HPLC method with diode array and ESI MS detection was developed for the analysis of the derivatized substances. ... |
|
|
Enantioselective absorption of chirally doped liquid crystalline networks studied by the use of an electronic microbalance.
J. Phys. Chem. B 111(31) , 9239-43, (2007) A polydomain cholesteric elastomer was obtained by cross-linking a nematic side-chain polysiloxane in the presence of a chiral dopant. After extraction of the chiral dopant, sorption experiments were performed, by the use of an electronic microbalance, in the... |
|
|
VCD study of alpha-methylbenzyl amine derivatives: detection of the unchanged chiral motif.
Chirality 22(8) , 754-61, (2010) Chiral alpha-methylbenzyl amine is a well known and often used chiral auxiliary, e.g., in the resolution of racemates or asymmetric catalysis. In this work, alpha-methylbenzyl amine and its derivatives N,alpha-dimethylbenzyl amine, N,N,alpha-trimethylbenzyl a... |