| Structure | Name/CAS No. | Articles |
|---|---|---|
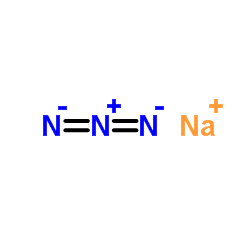 |
Sodium azide
CAS:26628-22-8 |
|
 |
Potassium bromide
CAS:7758-02-3 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
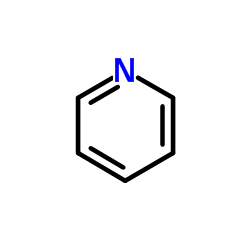 |
Pyridine
CAS:110-86-1 |
|
 |
HEPES
CAS:7365-45-9 |
|
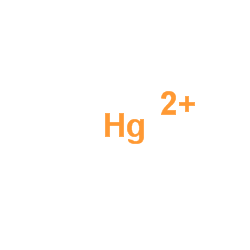 |
mercury(II) cation
CAS:7439-97-6 |
|
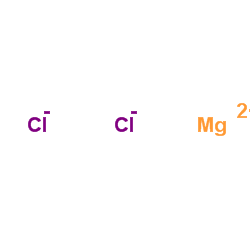 |
Magnesium choride
CAS:7786-30-3 |
|
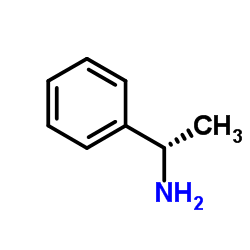 |
(R)-(+)-1-Phenylethylamine
CAS:3886-69-9 |
|
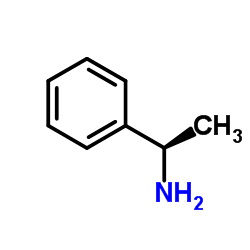 |
L-1-Phenylethylamine
CAS:2627-86-3 |
|
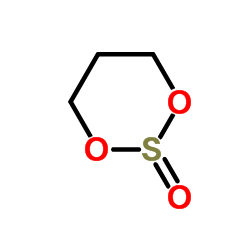 |
1,3,2-Dioxathiane 2-oxide
CAS:4176-55-0 |