4-Piperidinol
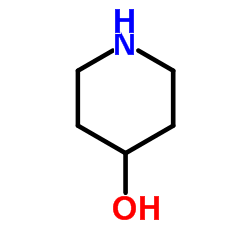
4-Piperidinol structure
|
Common Name | 4-Piperidinol | ||
|---|---|---|---|---|
| CAS Number | 5382-16-1 | Molecular Weight | 101.147 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 222.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C5H11NO | Melting Point | 86-90 °C | |
| MSDS | Chinese USA | Flash Point | 107.8±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis of novel IP agonists via N-aminoethyl cyclic amines prepared by decarboxylative ring-opening reactions.
Molecules 17(2) , 1233-46, (2012) An efficient synthesis of a highly potent and selective IP (PGI(2) receptor) agonist that is not structurally analogous to PGI(2) is described. This synthesis is accomplished through the following key steps: Nucleophilic ring-opening of 3-(4-chlorophenyl)-oxa... |
|
|
N- versus O-arylation of aminoalcohols: orthogonal selectivity in copper-based catalysts.
J. Am. Chem. Soc. 129 , 3490, (2007)
|
|
|
The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-Hydroxypiperidine by density functional method.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 75(3) , 941-52, (2010) In this work, we report a combined experimental and theoretical study on molecular structure, vibrational spectra, NBO and UV-spectral analysis of 4-Hydroxypiperidine (4-HP). The FT-IR solid phase (4000-400 cm(-1)), FT-IR gas phase (5000-400 cm(-1)) and FT-Ra... |
|
|
Investigation of 4-piperidinols as novel H3 antagonists.
Bioorg. Med. Chem. Lett. 20(21) , 6246-9, (2010) Compounds containing a substituted 4-piperidinol core have been found to be potent antagonists of the human H(3) receptor. The compounds exhibited up to a 60-fold preference for inhibiting the human H(3) receptor over the mouse and showed a low binding affini... |