Nestoron
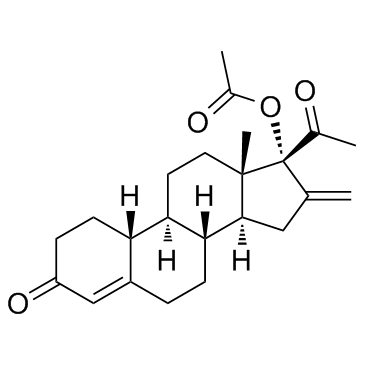
Nestoron structure
|
Common Name | Nestoron | ||
|---|---|---|---|---|
| CAS Number | 7759-35-5 | Molecular Weight | 370.48200 | |
| Density | 1.15g/cm3 | Boiling Point | 499.3ºC at 760mmHg | |
| Molecular Formula | C23H30O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 216.2ºC | |
|
Nestorone: clinical applications for contraception and HRT.
Steroids 68(10-13) , 907-13, (2003) The 19-nor derivatives of progesterone are referred to as "pure" progestational molecules as they bind almost exclusively to the progesterone receptor (PR) without interfering with receptors of other steroids. In this category is Nestorone, which has strong p... |
|
|
Recent developments in contraceptive implants at the Population Council.
Contraception 65(1) , 113-9, (2002) Development of contraceptive implant methods, when based on established or on new synthetic chemical entities, is a decadal or multi-decadal process. The process often requires the cooperation of numerous investigators for laboratory work, for early Phase II ... |
|
|
Central modifications of allopregnanolone and beta-endorphin following subcutaneous administration of Nestorone.
J. Steroid Biochem. Mol. Biol. 116(1-2) , 15-20, (2009) The aim of the present study was to evaluate the potential action of Nestorone (alone or in combination with estradiol valerate) on the level of allopregnanolone and of the opioid beta-endorphin in selected brain areas. Wistar ovariectomized rats were given 0... |
|
|
Nestorone progestin. The ideal progestin for use in controlled release delivery systems.
Ann. N. Y. Acad. Sci. 828 , 38-46, (1997)
|
|
|
Steroidal contraceptive vaginal rings.
Int. J. Clin. Pract. 57(5) , 392-5, (2003) The development of steroid-releasing vaginal rings over the past three decades is reviewed to illustrate the role of this device as an effective hormonal contraceptive for women. Vaginal rings are made of polysiloxane rubber or ethylene-vinyl-acetate copolyme... |
|
|
Discriminant analysis of the metabolic effects of a new combined contraceptive vaginal ring containing Nestorone/EE vs. a second-generation oral contraceptive containing levonorgestrel/EE.
Contraception 86(3) , 231-7, (2012) Discriminant analysis (DA) was performed on data of two combined hormonal contraceptives (CHC) differing in estrogen ratio to explore whether a combination of variables rather than a single variable distinguishes CHCs better.Data were used of a parallel study... |
|
|
The effect of Nestorone on gonadotropic cells in pituitary of rats.
Contraception 69(6) , 505-11, (2004) The implant containing Nestorone is a promising long-acting contraceptive especially suitable for lactating women. In this study, two experiments were designed to observe the effect of Nestorone on the gonadotropic cells in pituitary of rats for analyzing its... |
|
|
Enlarged ovarian follicles in users of a levonorgestrel-releasing intrauterine system and contraceptive implant.
J. Reprod. Med. 48(8) , 637-40, (2003) To evaluate the prevalence of enlarged ovarian follicles among users of a 20 micrograms/d levonorgestrel-releasing intrauterine system (Mirena, Leiras Oy, Turku, Finland), of subdermal contraceptive implants releasing Nestorone (Population Council, New York, ... |
|
|
Single-dose pharmacokinetics of Nestorone, a potential female-contraceptive.
Steroids 75(3) , 252-64, (2010) A synthetic progestin Nestorone is being developed for female-contraception. This study was conducted to determine the distribution, metabolism, and excretion of tritium-labeled Nestorone ((3)H Nestorone) in adult female rats. Rats were injected subcutaneousl... |
|
|
An initial pharmacokinetic study with a Metered Dose Transdermal Systemfor delivery of the progestogen Nestorone as a possible future contraceptive.
Contraception 76(6) , 432-8, (2007) Transdermal delivery of steroids is gaining popularity for contraception and hormone replacement therapy. This study aimed to test metered spray delivery of a precise dosage of Nestorone (NES) progestogen as a possible transdermal progestogen-only contracepti... |