Recent developments in contraceptive implants at the Population Council.
Irving Sivin, Alfred Moo-Young
Index: Contraception 65(1) , 113-9, (2002)
Full Text: HTML
Abstract
Development of contraceptive implant methods, when based on established or on new synthetic chemical entities, is a decadal or multi-decadal process. The process often requires the cooperation of numerous investigators for laboratory work, for early Phase II trials, for dose-finding trials, and for Phase III clinical trials. The Phase III work also requires cooperation with a commercial manufacturer and potential distributor of the product. The Population Council has recently completed developmental work on two levonorgestrel-releasing implants, with filings to regulatory agencies that support extended use of Jadelle implants for 5 years and Norplant implants for 7 years. When the developmental process includes establishing the clinical properties of a molecule not yet approved by regulatory agencies, the minimum development time appears to be two decades. The status and rationale of studies of a new Nestorone-releasing, single implant developed by the Population Council for a period of use of 2 years are presented.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
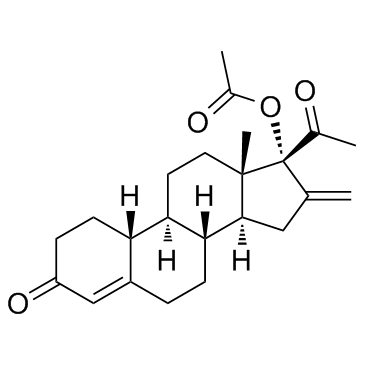 |
Nestoron
CAS:7759-35-5 |
C23H30O4 |
|
Nestorone: clinical applications for contraception and HRT.
2003-11-01 [Steroids 68(10-13) , 907-13, (2003)] |
|
Central modifications of allopregnanolone and beta-endorphin...
2009-08-01 [J. Steroid Biochem. Mol. Biol. 116(1-2) , 15-20, (2009)] |
|
Nestorone progestin. The ideal progestin for use in controll...
1997-09-26 [Ann. N. Y. Acad. Sci. 828 , 38-46, (1997)] |
|
Steroidal contraceptive vaginal rings.
2003-06-01 [Int. J. Clin. Pract. 57(5) , 392-5, (2003)] |
|
Discriminant analysis of the metabolic effects of a new comb...
2012-09-01 [Contraception 86(3) , 231-7, (2012)] |