Tosyl cyanide
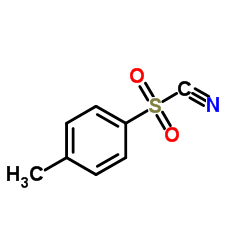
Tosyl cyanide structure
|
Common Name | Tosyl cyanide | ||
|---|---|---|---|---|
| CAS Number | 19158-51-1 | Molecular Weight | 181.212 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 289.8±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H7NO2S | Melting Point | 47-50 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 125.5±21.5 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
2-azabicyclo[2.2.2]octa-3,5-dione via a nitrile Diels-Alder reaction.
J. Org. Chem. 68(21) , 8256-7, (2003) The hetero-Diels-Alder reaction of an electron-deficient nitrile, p-toluenesulfonyl cyanide, with the silyl enol ether of cyclohexenone produced a hydrolytically sensitive [4 + 2] adduct in good yield. Use of Mander's reagent, ethyl cyanoformate, with the sam... |
|
|
Radical-mediated alkenylation, alkynylation, methanimination, and cyanation of B-alkylcatecholboranes.
Angew. Chem. Int. Ed. Engl. 45 , 5847, (2006)
|
|
|
Synthesis , 2551, (2006)
|
|
|
Preparation of polyfunctional nitriles by the cyanation of functionalized organozinc halides with p-toluenesulfonyl cyanide. Klement I, et al.
Tetrahedron Lett. 34(29) , 4623-26, (1993)
|
|
|
O-Sulfinylation of alcohols with methanesulfonyl cyanide or p-toluenesulfonyl cyanide. Barton DHR, et al.
Tetrahedron 47(44) , 9167-78, (1991)
|
|
|
A click chemistry approach to tetrazoles by Huisgen 1,3-dipolar cycloaddition: synthesis of 5-acyltetrazoles from azides and acyl cyanides.
Angew. Chem. Int. Ed. Engl. 41(12) , 2113-6, (2002)
|