| Structure | Name/CAS No. | Articles |
|---|---|---|
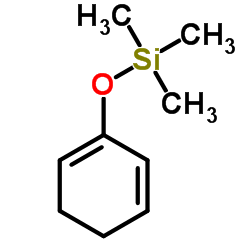 |
(1,5-Cyclohexadien-1-yloxy)(trimethyl)silane
CAS:54781-19-0 |
|
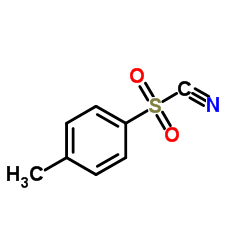 |
Tosyl cyanide
CAS:19158-51-1 |