(-)-Fenchone
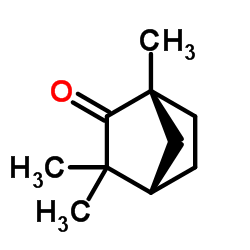
(-)-Fenchone structure
|
Common Name | (-)-Fenchone | ||
|---|---|---|---|---|
| CAS Number | 7787-20-4 | Molecular Weight | 152.233 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 193.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H16O | Melting Point | 5ºC | |
| MSDS | Chinese USA | Flash Point | 52.8±0.0 °C | |
| Symbol |

GHS02 |
Signal Word | Warning | |
|
[Chemical composition of essential oil obtained from Romanian fennel fruits].
Rev. Med. Chir. Soc. Med. Nat. Iasi. 115(2) , 590-4, (2011) For therapeutical purposes, fennel (Foeniculum vulgare Mill.), an important aromatic plant, is used for its expectorant, antispasmodic, carminative and diuretic properties. The chemical composition, especially of the volatile fraction, depends on the origin o... |
|
|
Biosynthesis of monoterpenes: conversion of the acyclic precursors geranyl pyrophosphate and neryl pyrophosphate to the rearranged monoterpenes fenchol and fenchone by a soluble enzyme preparation from fennel (Foeniculum vulgare).
Arch. Biochem. Biophys. 200(2) , 524-33, (1980)
|
|
|
Kinetics and mechanisms of the tropospheric reactions of menthol, borneol, fenchol, camphor, and fenchone with hydroxyl radicals (OH) and chlorine atoms (Cl).
J. Phys. Chem. A 116(16) , 4097-107, (2012) Relative kinetic techniques have been used to measure the rate coefficients for the reactions of oxygenated terpenes (menthol, borneol, fenchol, camphor, and fenchone) and cyclohexanol with hydroxyl radicals (OH) and chlorine atoms (Cl) at 298 ± 2 K and atmos... |
|
|
Comparative essential oil composition and antifungal effect of bitter fennel (Foeniculum vulgare ssp. piperitum) fruit oils obtained during different vegetation.
J. Med. Food 9(4) , 552-61, (2006) The chemical composition of the flower and unripe and ripe fruits from fennel (bitter) (Foeniculum vulgare ssp. piperitum) has been examined by gas chromatography and gas chromatography-mass spectrometry. The main identified components of the flower and unrip... |
|
|
Long-term stability of thujone, fenchone, and pinocamphone in vintage preban absinthe.
J. Agric. Food Chem. 57(7) , 2782-5, (2009) Research was conducted to ascertain whether analyses of vintage absinthe samples represent their original composition in the early 1900s. Absinthe stored in traditional green glass bottles and irradiated with ultraviolet light for up to 200 h exhibited unchan... |
|
|
Asymmetric electrophilic amination of various carbon nucleophiles with enantiomerically pure chiral N-h oxaziridines derived from camphor and fenchone.
J. Org. Chem. 67(22) , 7787-96, (2002) The first two stable enantiomerically pure chiral N-H oxaziridines, derived from camphor and fenchone, are shown to act as electrophilic sources of nitrogen upon reaction with various carbon nucleophiles. Nitrogen is transferred, together with the camphor/fen... |
|
|
Metabolism of (-)-fenchone by CYP2A6 and CYP2B6 in human liver microsomes.
Xenobiotica 37(2) , 194-204, (2007) The in vitro metabolism of (-)-fenchone was examined in human liver microsomes and recombinant enzymes. The biotransformation of (-)-fenchone was investigated by gas chromatography-mass spectrometry. (-)-Fenchone was found to be oxidized to 6-exo-hydroxyfench... |
|
|
Near-infrared analysis of fennel (Foeniculum vulgare Miller) on different spectrometers--basic considerations for a reliable network.
Phytochem. Anal. 14(5) , 285-9, (2003) The aim of this study was to investigate the accuracy and transferability of near-infrared (NIR) calibrations for estimating the content and composition of the volatile fraction in fennel fruits (Foeniculum vulgare Miller) as an example of medicinal and spice... |
|
|
Role of protein and substrate dynamics in catalysis by Pseudomonas putida cytochrome P450cam.
Biochemistry 41(49) , 14499-508, (2002) The role of protein structural flexibility and substrate dynamics in catalysis by cytochrome P450 enzymes is an area of current interest. We have addressed these in cytochrome P450(cam) (P450(cam)) and its Y96A mutant with camphor and its related compounds us... |
|
|
Metabolism of (+)-fenchone by CYP2A6 and CYP2B6 in human liver microsomes.
Biol. Pharm. Bull. 29(12) , 2354-8, (2006) The in vitro metabolism of (+)-fenchone was examined in human liver microsomes and recombinant enzymes. Biotransformation of (+)-fenchone was investigated by gas chromatography-mass spectrometry. (+)-Fenchone was found to be oxidized to 6-exo-hydroxyfenchone,... |