| Structure | Name/CAS No. | Articles |
|---|---|---|
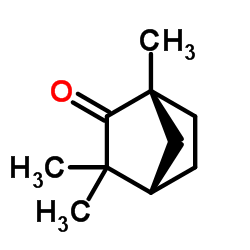 |
(-)-Fenchone
CAS:7787-20-4 |
|
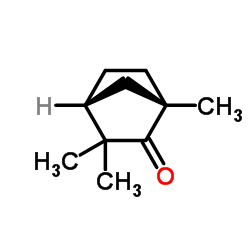 |
1,3,3-Trimethyl-2-norbornanone
CAS:4695-62-9 |