Manganese dioxide
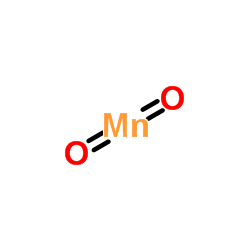
Manganese dioxide structure
|
Common Name | Manganese dioxide | ||
|---|---|---|---|---|
| CAS Number | 1313-13-9 | Molecular Weight | 86.937 | |
| Density | 5.02 | Boiling Point | N/A | |
| Molecular Formula | MnO2 | Melting Point | 535ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 535ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Shogaol-huprine hybrids: dual antioxidant and anticholinesterase agents with β-amyloid and tau anti-aggregating properties.
Bioorg. Med. Chem. 22(19) , 5298-307, (2014) Multitarget compounds are increasingly being pursued for the effective treatment of complex diseases. Herein, we describe the design and synthesis of a novel class of shogaol-huprine hybrids, purported to hit several key targets involved in Alzheimer's diseas... |
|
|
Fast and stable redox reactions of MnO₂/CNT hybrid electrodes for dynamically stretchable pseudocapacitors.
Nanoscale 7 , 11626-32, (2015) Pseudocapacitors, which are energy storage devices that take advantage of redox reactions to store electricity, have a different charge storage mechanism compared to lithium-ion batteries (LIBs) and electric double-layer capacitors (EDLCs), and they could rea... |
|
|
A highly efficient polysulfide mediator for lithium-sulfur batteries.
Nat. Commun. 6 , 5682, (2015) The lithium-sulfur battery is receiving intense interest because its theoretical energy density exceeds that of lithium-ion batteries at much lower cost, but practical applications are still hindered by capacity decay caused by the polysulfide shuttle. Here w... |
|
|
Determination of polyphenolic compounds in Cirsium palustre (L.) extracts by high performance liquid chromatography with chemiluminescence detection.
Talanta 133 , 38-44, (2014) The first method for the simultaneous determination of polyphenolic antioxidants in extracts from leaves of Cirsium palustre based on high performance liquid chromatography combined with flow injection chemiluminescence detection (HPLC-FI-CL) has been develop... |
|
|
High-performance hybrid oxide catalyst of manganese and cobalt for low-pressure methanol synthesis.
Nat. Commun. 6 , 6538, (2015) Carbon dioxide capture and use as a carbon feedstock presents both environmental and industrial benefits. Here we report the discovery of a hybrid oxide catalyst comprising manganese oxide nanoparticles supported on mesoporous spinel cobalt oxide, which catal... |
|
|
Carotenoid profile of tomato sauces: effect of cooking time and content of extra virgin olive oil.
Int. J. Mol. Sci. 16 , 9588-99, (2015) The consumption of carotenoid-rich vegetables such as tomatoes and tomato sauces is associated with reduced risk of several chronic diseases. The predominant carotenoids in tomato products are in the (all-E) configuration, but (Z) isomers can be formed during... |
|
|
Development of optically transparent water oxidation catalysts using manganese pyrophosphate compounds.
J. Photochem. Photobiol. B, Biol. 152 , 139-45, (2015) One challenge in artificial photosynthetic systems is the development of active oxygen evolution catalysts composed of abundant elements. The oxygen evolution activities of manganese pyrophosphate compounds were examined in electrochemical and photochemical e... |
|
|
Rhodium(I)-catalyzed regiospecific dimerization of aromatic acids: two direct ch bond activations in water.
Angew. Chem. Int. Ed. Engl. 54 , 5718-21, (2015) 2,2'-Diaryl acids are key building blocks for some of the most important and high-performance polymers such as polyesters and polyamides (imides), as well as structural motifs of MOFs (metal-organic frameworks) and biological compounds. In this study, a direc... |
|
|
The water catalysis at oxygen cathodes of lithium-oxygen cells.
Nat. Commun. 6 , 7843, (2015) Lithium-oxygen cells have attracted extensive interests due to their high theoretical energy densities. The main challenges are the low round-trip efficiency and cycling instability over long time. However, even in the state-of-the-art lithium-oxygen cells th... |
|
|
Effectively suppressing dissolution of manganese from spinel lithium manganate via a nanoscale surface-doping approach.
Nat. Commun. 5 , 5693, (2014) The capacity fade of lithium manganate-based cells is associated with the dissolution of Mn from cathode/electrolyte interface due to the disproportionation reaction of Mn(III), and the subsequent deposition of Mn(II) on the anode. Suppressing the dissolution... |