| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
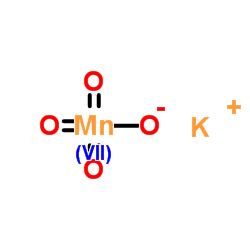 |
Potassium permanganate
CAS:7722-64-7 |
|
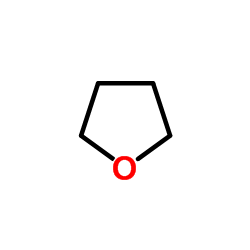 |
thf
CAS:109-99-9 |
|
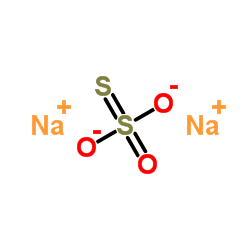 |
Sodium thiosulfate
CAS:7772-98-7 |
|
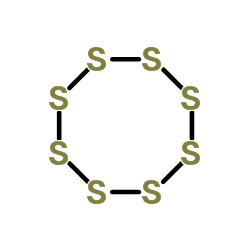 |
Sulfur
CAS:7704-34-9 |
|
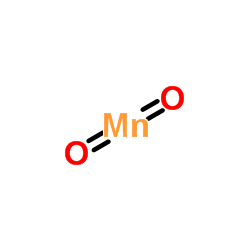 |
Manganese dioxide
CAS:1313-13-9 |
|
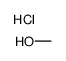 |
HYDROGEN CHLORIDE ~1.25 M IN METHANOL, 250 ML
CAS:132228-87-6 |