Alpha-Angelica lactone
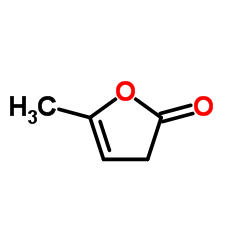
Alpha-Angelica lactone structure
|
Common Name | Alpha-Angelica lactone | ||
|---|---|---|---|---|
| CAS Number | 591-12-8 | Molecular Weight | 98.100 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 249.4±9.0 °C at 760 mmHg | |
| Molecular Formula | C5H6O2 | Melting Point | 13-17 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 68.3±0.0 °C | |
|
Characterization of the PON1 active site using modeling simulation, in relation to PON1 lactonase activity
Bioorg. Med. Chem. 16 , 7504-9, (2008) Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters an... |
|
|
Enhancement of rat hepatic and gastrointestinal glutathione and glutathione S-transferases by alpha-angelicalactone and flavone.
Carcinogenesis 16(3) , 607-12, (1995) The naturally occurring anticarcinogens flavone and alpha-angelicalactone incorporated separately and simultaneously in the diet at 0.5, 0.1, 0.05 and 0.01% w/w, were studied with respect to their effects on oesophageal, gastric, intestinal, colonic and hepat... |
|
|
Catalytic asymmetric vinylogous Mannich-type (AVM) reaction of nonactivated α-angelica lactone.
Org. Lett. 13(12) , 3056-9, (2011) A direct highly diastereo- and enantioselective asymmetric vinylogous Mannich-type (AVM) reaction of aldimines with nonactivated natural α-angelica lactone has been successfully developed. It was demonstrated that the nonactivated natural α-angelica lactone i... |
|
|
Effect of butylated hydroxyanisole, alpha-angelica lactone, and beta-naphthoflavone on benzo(alpha)pyrene:DNA adduct formation in vivo in the forestomach, lung, and liver of mice.
Cancer Res. 42(4) , 1199-204, (1982) The effects of alpha-angelica lactone (alpha-AL), butylated hydroxyanisole (BHA), and beta-naphthoflavone (beta-NF) on the amount of benzo(alpha)pyrene (BP) metabolite:DNA adducts formed in the forestomach, lung, and liver of ICR/Ha mice were investigated 48 ... |
|
|
Asymmetric assembly of 2-oxindole and α-angelica lactone units to construct vicinal quaternary chiral centers.
Chem. Commun. (Camb.) 48(18) , 2439-41, (2012) The first organocatalytic asymmetric assembly of Morita-Baylis-Hillman carbonates of isatins and α-angelica lactone has been studied, affording multifunctional products containing two valuable pharmacophores and vicinal quaternary chiral centers in high stere... |