| Structure | Name/CAS No. | Articles |
|---|---|---|
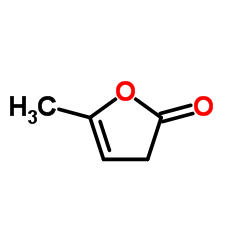 |
Alpha-Angelica lactone
CAS:591-12-8 |
Lin Zhou, Lili Lin, Jie Ji, Mingsheng Xie, Xiaohua Liu, Xiaoming Feng
Index: Org. Lett. 13(12) , 3056-9, (2011)
Full Text: HTML
A direct highly diastereo- and enantioselective asymmetric vinylogous Mannich-type (AVM) reaction of aldimines with nonactivated natural α-angelica lactone has been successfully developed. It was demonstrated that the nonactivated natural α-angelica lactone is a useful vinylogous nucleophile to give the chiral δ-amino γ,γ-disubstituted butenolide carbonyl derivatives. The N,N'-dioxide L2-Sc(III) complex is efficient toward the obtention of a range of corresponding products with adjacent quaternary and tertiary stereocenters in excellent dr and ee values.© 2011 American Chemical Society
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Alpha-Angelica lactone
CAS:591-12-8 |
C5H6O2 |
|
Characterization of the PON1 active site using modeling simu...
2008-01-01 [Bioorg. Med. Chem. 16 , 7504-9, (2008)] |
|
Enhancement of rat hepatic and gastrointestinal glutathione ...
1995-03-01 [Carcinogenesis 16(3) , 607-12, (1995)] |
|
Effect of butylated hydroxyanisole, alpha-angelica lactone, ...
1982-04-01 [Cancer Res. 42(4) , 1199-204, (1982)] |
|
Asymmetric assembly of 2-oxindole and α-angelica lactone uni...
2012-02-28 [Chem. Commun. (Camb.) 48(18) , 2439-41, (2012)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved