Dibenzyl-(E)-diazen-1,2-dicarboxylat
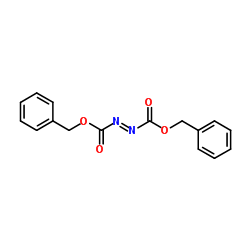
Dibenzyl-(E)-diazen-1,2-dicarboxylat structure
|
Common Name | Dibenzyl-(E)-diazen-1,2-dicarboxylat | ||
|---|---|---|---|---|
| CAS Number | 2449-05-0 | Molecular Weight | 298.293 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 456.8±34.0 °C at 760 mmHg | |
| Molecular Formula | C16H14N2O4 | Melting Point | 43-47 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 203.0±20.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Cu- and Pd-catalyzed asymmetric one-pot tandem addition-cyclization reaction of 2-(2',3'-alkadienyl)-beta-keto esters, organic halides, and dibenzyl azodicarboxylate: an effective protocol for the enantioselective synthesis of pyrazolidine derivatives.
Org. Lett. 6(13) , 2193-6, (2004) [reaction: see text] Optically active pyrazolidine derivatives have been constructed by the Cu- and Pd-catalyzed asymmetric one-pot tandem addition-cyclization reaction of 2-(2',3'-dienyl)-beta-ketoesters, organic halides, and dibenzyl azodicarboxylate. The a... |
|
|
General synthesis of C-glycosyl amino acids via proline-catalyzed direct electrophilic alpha-amination of C-glycosylalkyl aldehydes.
Org. Lett. 10(20) , 4485-8, (2008) Non-natural axially and equatorially linked C-glycosyl alpha-amino acids (glycines, alanines, and CH2-serine isosteres) with either S or R alpha-configuration were prepared by D- and L-proline-catalyzed (de >95%) alpha-amination of C-glycosylalkyl aldehydes u... |
|
|
[4+ 2] Cycloaddition reaction of dibenzyl azodicarboxylate and glycals. Leblanc Y, et al.
J. Am. Chem. Soc. 111(8) , 2995-3000, (1989)
|