Dibenzyl-(E)-diazen-1,2-dicarboxylat
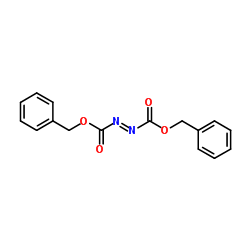
Dibenzyl-(E)-diazen-1,2-dicarboxylat structure
|
Common Name | Dibenzyl-(E)-diazen-1,2-dicarboxylat | ||
|---|---|---|---|---|
| CAS Number | 2449-05-0 | Molecular Weight | 298.293 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 456.8±34.0 °C at 760 mmHg | |
| Molecular Formula | C16H14N2O4 | Melting Point | 43-47 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 203.0±20.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | Dibenzyl azodicarboxylate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 456.8±34.0 °C at 760 mmHg |
| Melting Point | 43-47 °C(lit.) |
| Molecular Formula | C16H14N2O4 |
| Molecular Weight | 298.293 |
| Flash Point | 203.0±20.1 °C |
| Exact Mass | 298.095367 |
| PSA | 77.32000 |
| LogP | 4.34 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.568 |
| Storage condition | 0-6°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | F:Flammable;Xi:Irritant; |
| Risk Phrases | R11;R36/37/38 |
| Safety Phrases | S26-S37/39-S16 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2927000090 |
|
~% 
Dibenzyl-(E)-di... CAS#:2449-05-0 |
| Literature: Journal of the Chemical Society, , p. 2089,2093 |
|
~% 
Dibenzyl-(E)-di... CAS#:2449-05-0 |
| Literature: Journal of the Chemical Society, , p. 2089,2093 |
| HS Code | 2927000090 |
|---|---|
| Summary | 2927000090 other diazo-, azo- or azoxy-compounds。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Cu- and Pd-catalyzed asymmetric one-pot tandem addition-cyclization reaction of 2-(2',3'-alkadienyl)-beta-keto esters, organic halides, and dibenzyl azodicarboxylate: an effective protocol for the enantioselective synthesis of pyrazolidine derivatives.
Org. Lett. 6(13) , 2193-6, (2004) [reaction: see text] Optically active pyrazolidine derivatives have been constructed by the Cu- and Pd-catalyzed asymmetric one-pot tandem addition-cyclization reaction of 2-(2',3'-dienyl)-beta-ketoes... |
|
|
General synthesis of C-glycosyl amino acids via proline-catalyzed direct electrophilic alpha-amination of C-glycosylalkyl aldehydes.
Org. Lett. 10(20) , 4485-8, (2008) Non-natural axially and equatorially linked C-glycosyl alpha-amino acids (glycines, alanines, and CH2-serine isosteres) with either S or R alpha-configuration were prepared by D- and L-proline-catalyz... |
|
|
[4+ 2] Cycloaddition reaction of dibenzyl azodicarboxylate and glycals. Leblanc Y, et al.
J. Am. Chem. Soc. 111(8) , 2995-3000, (1989)
|
| Dibenzyl azodicarbox |
| EINECS 219-508-0 |
| dibenzyl diazodicarboxylate |
| Azodicarboxylic Acid Dibenzyl Ester |
| Dibenzyl Azodicarboxylate |
| Dibenzyl (E)-diazene-1,2-dicarboxylate |
| Azodiformic acid dibenzyl ester |
| dibenzyl diazenedicarboxylate |
| Dibenzyl diazene-1,2-dicarboxylate |
| MFCD00016737 |
| di-benzyl azodicarboxylate |
| 1,2-Diazenedicarboxylic acid, bis(phenylmethyl) ester, (E)- |
| Dibenzyl-(E)-diazen-1,2-dicarboxylat |
| Dibenzyl (E)-1,2-diazenedicarboxylate |
| DBAD |
 CAS#:65632-62-4
CAS#:65632-62-4 CAS#:816454-25-8
CAS#:816454-25-8
