Ganirelix Acetate
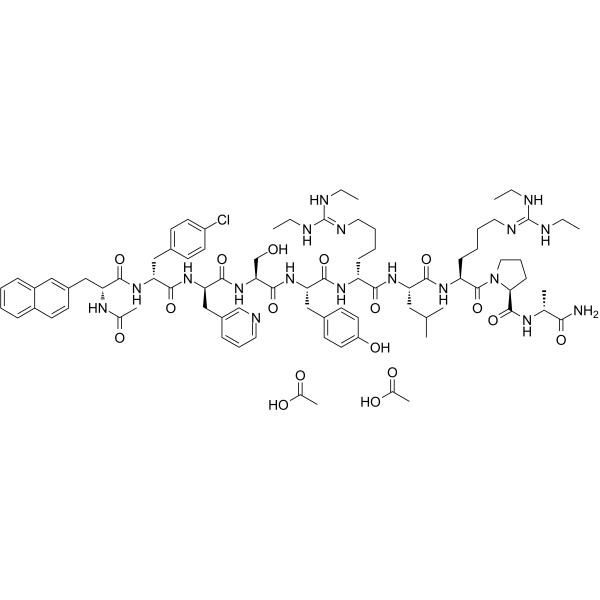
Ganirelix Acetate结构式

|
常用名 | Ganirelix Acetate | 英文名 | Ganirelix Acetate |
|---|---|---|---|---|
| CAS号 | 129311-55-3 | 分子量 | 1690.423 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C84H121ClN18O17 | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | N/A | |
| 符号 |

GHS07 |
信号词 | Warning |
Ganirelix Acetate用途醋酸甘乃瑞利(Ganirelix醋酸盐,Ganirest)是一种可注射竞争性促性腺激素释放激素(GnRH)拮抗剂。甘乃瑞利醋酸盐直接与内源性分子竞争受体结合,并导致雌二醇水平迅速降低。甘乃瑞利醋酸盐可用于研究卵巢过度刺激综合征(OHSS)[1]。 |
| 英文名 | Ganirelix acetate |
|---|---|
| 英文别名 | 更多 |
| 描述 | 醋酸甘乃瑞利(Ganirelix醋酸盐,Ganirest)是一种可注射竞争性促性腺激素释放激素(GnRH)拮抗剂。甘乃瑞利醋酸盐直接与内源性分子竞争受体结合,并导致雌二醇水平迅速降低。甘乃瑞利醋酸盐可用于研究卵巢过度刺激综合征(OHSS)[1]。 |
|---|---|
| 相关类别 | |
| 靶点 |
GnRH[1] |
| 参考文献 |
| 分子式 | C84H121ClN18O17 |
|---|---|
| 分子量 | 1690.423 |
| 精确质量 | 1688.884521 |
| InChIKey | OVBICQMTCPFEBS-SATRDZAXSA-N |
| SMILES | CC(=O)O.CC(=O)O.CCNC(=NCCCCC(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(CO)NC(=O)C(Cc1cccnc1)NC(=O)C(Cc1ccc(Cl)cc1)NC(=O)C(Cc1ccc2ccccc2c1)NC(C)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCCN=C(NCC)NCC)C(=O)N1CCCC1C(=O)NC(C)C(N)=O)NCC |
| 储存条件 | -20°C,密闭,干燥 |
| 符号 |

GHS07 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H315-H319-H335 |
| 警示性声明 | P261-P305 + P351 + P338 |
| 危险品运输编码 | NONH for all modes of transport |
|
Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors.
Am. J. Physiol. Endocrinol. Metab. 305(7) , E818-25, (2013) Hyperandrogenism and vascular dysfunction often coexist in women with polycystic ovary syndrome (PCOS). We hypothesized that testosterone compromises cutaneous microvascular dilation in women with PCO... |
|
|
GnRH antagonist versus long GnRH agonist protocol in poor IVF responders: a randomized clinical trial.
Eur. J. Obstet. Gynecol. Reprod. Biol. 166(1) , 43-6, (2013) To compare the efficacy of the long GnRH agonist and the fixed GnRH antagonist protocols in IVF poor responders.This was a randomized controlled trial performed in the Iakentro IVF centre, Thessalonik... |
|
|
Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: an observational cohort study.
Reprod. Biol. Endocrinol. 10 , 69, (2012) Management of established severe OHSS requires prolonged hospitalization, occasionally in intensive care units, accompanied by multiple ascites punctures, correction of intravascular fluid volume and ... |
| 56U7906FQW |
| D-Alaninamide, N-acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-L-tyrosyl-N6-[bis(ethylamino)methylene]-D-lysyl-L-leucyl-N6-[bis(ethylamino)methylene]- ;L-lysyl-L-prolyl-, acetate (1:2) |
| Ganirelix acetate |
| UNII:56U7906FQW |
| N-Acetyl-3-(2-naphthyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-L-tyrosyl-N6-[bis(ethylamino)methylene]-D-lysyl-L-leucyl-N6-[bis(ethylamino)methylene]-L-lysyl-L-prolyl-D- ;alaninamide acetate (1:2) |


