Ganirelix Acetate
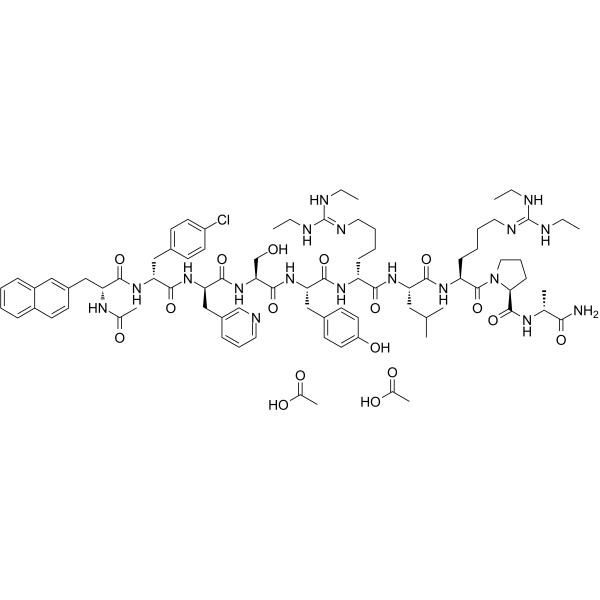
Ganirelix Acetate structure
|
Common Name | Ganirelix Acetate | ||
|---|---|---|---|---|
| CAS Number | 129311-55-3 | Molecular Weight | 1690.423 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C84H121ClN18O17 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Ganirelix AcetateGanirelix acetate (Ganirest) is an injectable competitive gonadotropin-releasing hormone (GnRH) antagonist. Ganirelix acetate directly competes against the endogenous molecule for receptor binding, and causes a rapid reduction in estradiol levels. Ganirelix acetate can be used for researching ovarian hyperstimulation syndrome (OHSS)[1]. |
| Name | Ganirelix acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Ganirelix acetate (Ganirest) is an injectable competitive gonadotropin-releasing hormone (GnRH) antagonist. Ganirelix acetate directly competes against the endogenous molecule for receptor binding, and causes a rapid reduction in estradiol levels. Ganirelix acetate can be used for researching ovarian hyperstimulation syndrome (OHSS)[1]. |
|---|---|
| Related Catalog | |
| Target |
GnRH[1] |
| References |
| Molecular Formula | C84H121ClN18O17 |
|---|---|
| Molecular Weight | 1690.423 |
| Exact Mass | 1688.884521 |
| InChIKey | OVBICQMTCPFEBS-SATRDZAXSA-N |
| SMILES | CC(=O)O.CC(=O)O.CCNC(=NCCCCC(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(CO)NC(=O)C(Cc1cccnc1)NC(=O)C(Cc1ccc(Cl)cc1)NC(=O)C(Cc1ccc2ccccc2c1)NC(C)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCCN=C(NCC)NCC)C(=O)N1CCCC1C(=O)NC(C)C(N)=O)NCC |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
|
Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors.
Am. J. Physiol. Endocrinol. Metab. 305(7) , E818-25, (2013) Hyperandrogenism and vascular dysfunction often coexist in women with polycystic ovary syndrome (PCOS). We hypothesized that testosterone compromises cutaneous microvascular dilation in women with PCO... |
|
|
GnRH antagonist versus long GnRH agonist protocol in poor IVF responders: a randomized clinical trial.
Eur. J. Obstet. Gynecol. Reprod. Biol. 166(1) , 43-6, (2013) To compare the efficacy of the long GnRH agonist and the fixed GnRH antagonist protocols in IVF poor responders.This was a randomized controlled trial performed in the Iakentro IVF centre, Thessalonik... |
|
|
Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: an observational cohort study.
Reprod. Biol. Endocrinol. 10 , 69, (2012) Management of established severe OHSS requires prolonged hospitalization, occasionally in intensive care units, accompanied by multiple ascites punctures, correction of intravascular fluid volume and ... |
| 56U7906FQW |
| D-Alaninamide, N-acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-L-tyrosyl-N6-[bis(ethylamino)methylene]-D-lysyl-L-leucyl-N6-[bis(ethylamino)methylene]- ;L-lysyl-L-prolyl-, acetate (1:2) |
| Ganirelix acetate |
| UNII:56U7906FQW |
| N-Acetyl-3-(2-naphthyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-L-tyrosyl-N6-[bis(ethylamino)methylene]-D-lysyl-L-leucyl-N6-[bis(ethylamino)methylene]-L-lysyl-L-prolyl-D- ;alaninamide acetate (1:2) |