| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
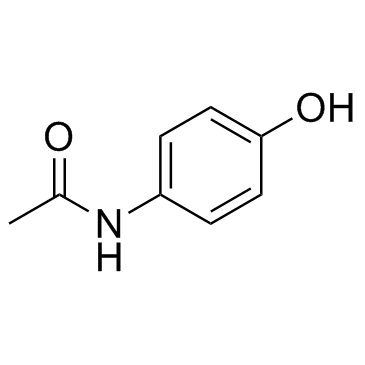 |
对乙酰氨基苯酚
CAS:103-90-2 |
|
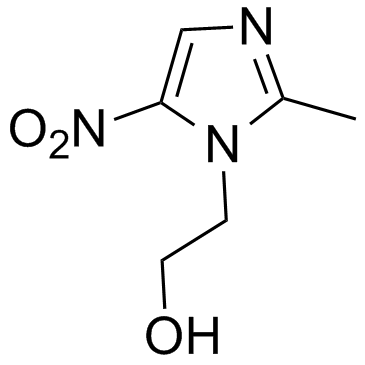 |
甲硝唑
CAS:443-48-1 |
|
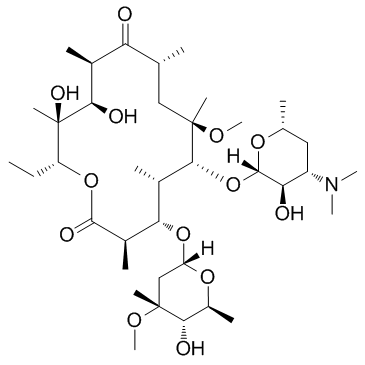 |
克拉霉素
CAS:81103-11-9 |
|
 |
地塞米松
CAS:50-02-2 |
|
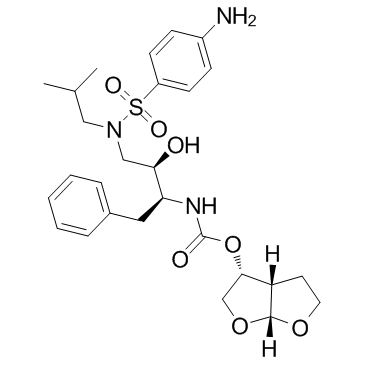 |
地瑞拉韦
CAS:206361-99-1 |
|
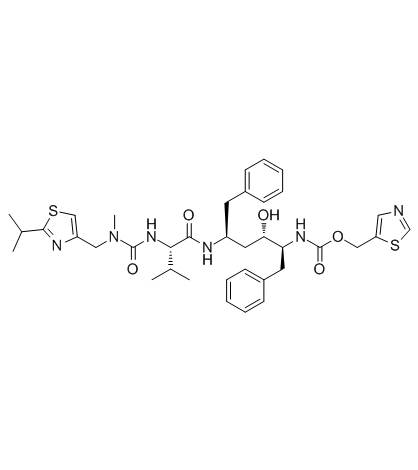 |
利托那韦
CAS:155213-67-5 |
|
 |
依法韦仑
CAS:154598-52-4 |
|
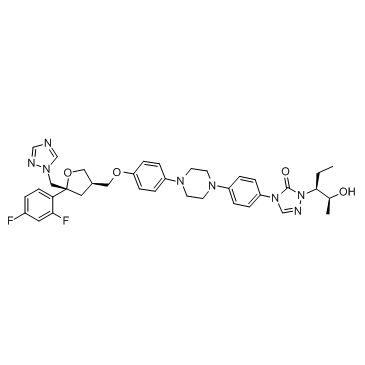 |
泊沙康唑
CAS:171228-49-2 |
|
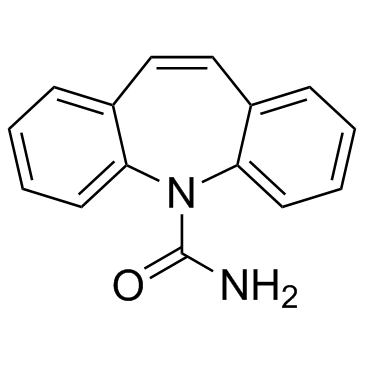 |
卡马西平
CAS:298-46-4 |