| Structure | Name/CAS No. | Articles |
|---|---|---|
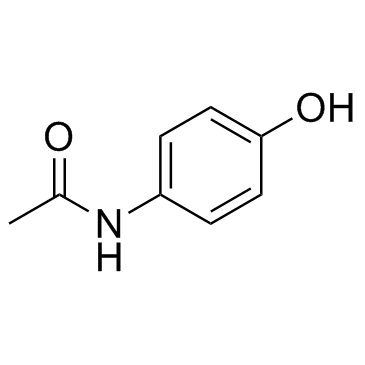 |
4-Acetamidophenol
CAS:103-90-2 |
|
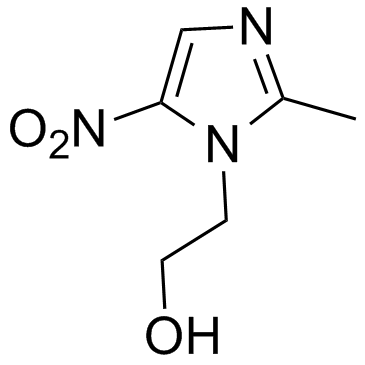 |
Metronidazole
CAS:443-48-1 |
|
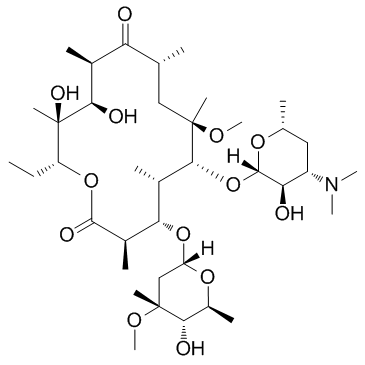 |
Clarithromycin
CAS:81103-11-9 |
|
 |
Dexamethasone
CAS:50-02-2 |
|
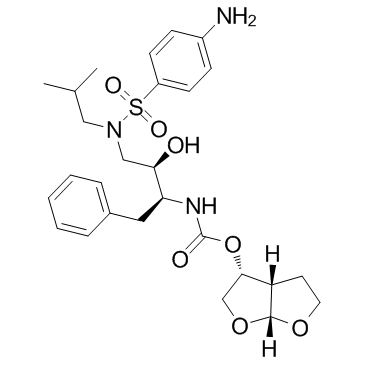 |
Darunavir
CAS:206361-99-1 |
|
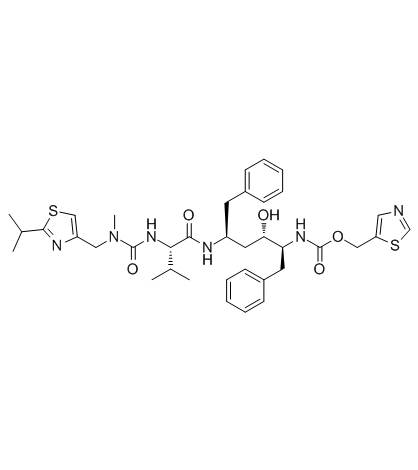 |
Ritonavir
CAS:155213-67-5 |
|
 |
Efavirenz
CAS:154598-52-4 |
|
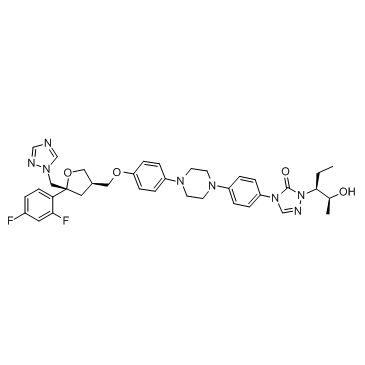 |
Posaconazole
CAS:171228-49-2 |
|
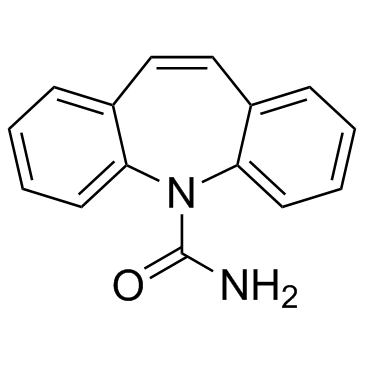 |
Carbamazepine
CAS:298-46-4 |