Posaconazole
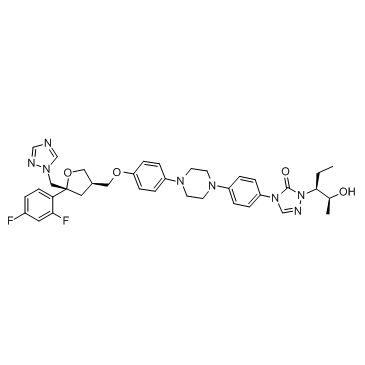
Posaconazole structure
|
Common Name | Posaconazole | ||
|---|---|---|---|---|
| CAS Number | 171228-49-2 | Molecular Weight | 700.777 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 850.7±75.0 °C at 760 mmHg | |
| Molecular Formula | C37H42F2N8O4 | Melting Point | 170-1720C | |
| MSDS | Chinese USA | Flash Point | 468.3±37.1 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of PosaconazolePosaconazole is a broad-spectrum, second generation, triazole compound with antifungal activity. |
| Name | posaconazole |
|---|---|
| Synonym | More Synonyms |
| Description | Posaconazole is a broad-spectrum, second generation, triazole compound with antifungal activity. |
|---|---|
| Related Catalog | |
| In Vitro | Posaconazole has potent trypanocidal activity. Amiodarone acts synergistically with Posaconazole. Posaconazole also affects and disrupts Ca2+ homeostasis in T. cruzi. Posaconazole blocks the biosynthesis of ergosterol, which is essential for parasite survival. Posaconazole has a clear, dose-dependent effect on proliferation of the epimastigote (extracellular) stages, with a minimal inhibitory concentration of 20 nM and an IC50 of 14 nM. Against the clinically relevant intracellular amastigote form of the parasite, Posaconazole is even more potent. Posaconazole has the minimal inhibitory concentration and IC50 values of 3 nM and 0.25 nM[1]. Posaconazole is active against isolates of Candida and Aspergillus spp. that exhibit resistance to Fluconazole, Voriconazole, and Amphotericin B and is much more active than the other triazoles against zygomycetes[2]. |
| In Vivo | Treatment of infected animals with amiodarone alone reduces parasitemia, increases survival 60 days pi (0% for untreated controls vs 40% for amiodarone-treated animals) and, when given in combination with Posaconazole, delays the development of parasitemia[1]. Coadministration of Posaconazole and Boost Plus increases drug exposure compared to the administration of Posaconazole alone in the fasted state. Food, particularly meals high in fat content, significantly increases Posaconazole bioavailability. Systemic exposure to Posaconazole increases 4- and 2.6-fold when it is consumed with a high-fat and nonfat meal, respectively[3]. Posaconazole and Amiodarone may constitute an effective anti-T. cruzi therapy with low side effect[4]. At twice-daily doses of ≥ 15 mg/kg of body weight, Posaconazole prolongs the survival of the mice and reduces tissue burden[5]. |
| Cell Assay | The epimastigote form of the parasite is cultivated in liver infusion tryptose medium,12 supplemented with 10% new born calf serum at 28°C with strong (120 rpm) agitation. Cultures are initiated at a cell density of 2×106 epimastigotes mL-1, and drugs are added at a cell density of 0.5−1.0 ×107 epimastigotes mL-1. Cell densities are measured by using an electronic particle counter as well as by direct counting with a hemocytometer. Cell viability is followed by Trypan blue exclusion, using light microscopy. Amastigotes are cultured in Vero cells maintained in minimal essential medium supplemented with 1% fetal calf serum in a humidified atmosphere (95% air−5% CO2) at 37°C, as described previously. Cells are infected with 10 tissue culture-derived trypomastigotes per cell for 2 h and then washed three times with phosphate-buffered saline (PBS) to remove nonadherent parasites. Fresh medium with and without drugs is added, and the cells are incubated for 96 h with a medium change at 48 h. The percent of infected cells and the numbers of parasites per cell are determined directly using light microscopy, and a statistical analysis of the results is carried out as described previously. IC50 values are calculated by nonlinear regression, using the program GraFit. Cytoplasmic free Ca2+ concentrations in control and drug-treated extracellular epimastigotes are determined by fluorimetric methods, using Fura-2, again as described previously. Subcellular Ca2+ levels and mitochondrial membrane potentials are monitored on individual Vero cells infected with T. cruzi amastigotes by using time-scan confocal microscopy, as described in detail elsewhere. Briefly, Vero cells heavily infected (72 h) with T. cruzi amastigotes are plated onto 22×40 mm glass coverslips (0.15 mm thickness) and incubated simultaneously with 10 μM cell-permeant Rhod-2 and 10 μg/mL Rhodamine-123 for 50 min at 37°C in culture medium and then washed and incubated with Ringer's solution, with or without amiodarone. Under the conditions used, fluorescence of Rhod-2 comes mainly from intracellular Ca2+-rich compartments, like mitochondria, since its low affinity for Ca2+ limits its fluorescence in the Ca2+-poor cytoplasm of the Vero cells or amastigotes. Rhodamine-123 is a mitochondrion-specific cationic dye, which distributes across the inner mitochondrial membranes strictly according to their membrane potential. |
| Animal Admin | In vivo studies are carried out by using the murine model of acute Chagas' disease in which female NMRI−IVIC mice (20−25 g) are infected with 105 or 103 bloodstream trypomastigotes and drug treatment is started 24 h later. Treatments are given for 30 consecutive days at 20 mg/kg/d for posaconazole (30 doses) and/or at 50 mg/kg every other day for amiodarone (15 doses). Negative controls (i.e. untreated animals) receive only the vehicle, while positive controls are treated with the anti-T. cruzi compound, nifurtimox, at 50 mg/kg/d for 30 days. Survival is followed daily and parasitemia weekly, the latter by direct microscopic examination. Animals are observed for 60 days postinfection, after which time parasitological cures are evaluated by using a combination of hemoculture, xenodiagnosis, and blood PCR tests. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 850.7±75.0 °C at 760 mmHg |
| Melting Point | 170-1720C |
| Molecular Formula | C37H42F2N8O4 |
| Molecular Weight | 700.777 |
| Flash Point | 468.3±37.1 °C |
| Exact Mass | 700.329712 |
| PSA | 115.70000 |
| LogP | 2.25 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.658 |
| Storage condition | -20°C Freezer |
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Hazard Codes | F,T |
| Risk Phrases | 11-23/24/25-39/23/24/25 |
| Safety Phrases | S61 |
| RIDADR | NONH for all modes of transport |
|
In vivo and in vitro acquisition of resistance to voriconazole by Candida krusei.
Antimicrob. Agents Chemother. 58(8) , 4604-11, (2014) Candida krusei is an important agent of opportunistic infections that often displays resistance to several antifungals. We describe here the in vivo acquisition of resistance to voriconazole (VRC) by ... |
|
|
European Confederation of Medical Mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe.
Eur. J. Clin. Microbiol. Infect. Dis. 33(9) , 1623-30, (2014) In order to better understand the epidemiology of fusariosis in Europe, a survey collecting information on the clinical characteristics of the patients infected by Fusarium as well as on the infecting... |
|
|
Simultaneous determination of seven azole antifungal drugs in serum by ultra-high pressure liquid chromatography and diode array detection.
Acta Clin. Belg. 69(1) , 53-61, (2014) Azole antifungals are a group of fungistatic agents that can be administered orally or parenterally. The determination of the concentrations of these antifungals (miconazole, fluconazole, ketoconazole... |
| 2,5-Anhydro-1,3,4-trideoxy-2-(2,4-difluorophenyl)-4-({4-[4-(4-{1-[(2S,3S)-2-hydroxy-3-pentanyl]-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl}phenyl)-1-piperazinyl]phenoxy}methyl)-1-(1H-1,2,4-triazol-1-yl)-D-threo-pentitol |
| 2,5-Anhydro-1,3,4-trideoxy-2-(2,4-difluorophenyl)-4-({4-[4-(4-{1-[(2S,3S)-2-hydroxypentan-3-yl]-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl}phenyl)piperazin-1-yl]phenoxy}methyl)-1-(1H-1,2,4-triazol-1-yl)-D-threo-pentitol |
| D-threo-Pentitol, 2,5-anhydro-1,3,4-trideoxy-2-C-(2,4-difluorophenyl)-4-[[4-[4-[4-[1-[(1S,2S)-1-ethyl-2-hydroxypropyl]-1,5-dihydro-5-oxo-4H-1,2,4-triazol-4-yl]phenyl]-1-piperazinyl]phenoxy]methyl]-1-(1H-1,2,4-triazol-1-yl)- |
| Pcz |
| EINECS 200-659-6 |
| Noxafil |
| HYDROXYPROPYL]-2,4-DIHYDRO-3H-1,2,4-TRIAZOL-3-ONE |
| Posaconazole for research |
| T6N DNTJ AR D- DT5NNVJ BY2&YQ1&& DR DO1- DT5OTJ BR BF DF& B1- AT5NN DNJ &&Stereoisomer |
| Pos |
| 2,5-Anhydro-1,3,4-trideoxy-2-(2,4-difluorophenyl)-4-({4-[4-(4-{1-[(1S,2S)-1-ethyl-2-hydroxypropyl]-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl}phenyl)piperazin-1-yl]phenoxy}methyl)-1-(1H-1,2,4-triazol-1-yl)-D-threo-pentitol |
| 4-{4-[4-(4-{[(3R,5R)-5-(2,4-difluorophényl)-5-(1H-1,2,4-triazol-1-ylméthyl)tétrahydrofuran-3-yl]méthoxy}phényl)pipérazin-1-yl]phényl}-2-[(1S,2S)-1-éthyl-2-hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one |
| 4-{4-[4-(4-{[(3R,5R)-5-(2,4-Difluorophenyl)-5-(1H-1,2,4-triazol-1-ylmethyl)tetrahydrofuran-3-yl]methoxy}phenyl)piperazin-1-yl]phenyl}-2-[(1S,2S)-1-ethyl-2-hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one |
| Posconazole |
| Posaconazole |
 CAS#:170985-86-1
CAS#:170985-86-1 CAS#:106-41-2
CAS#:106-41-2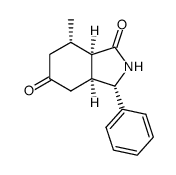 CAS#:454479-36-8
CAS#:454479-36-8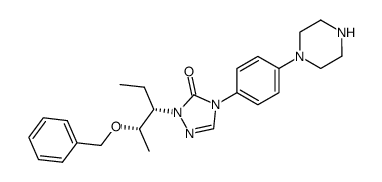 CAS#:454479-39-1
CAS#:454479-39-1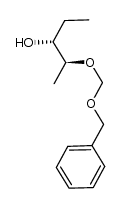 CAS#:171228-61-8
CAS#:171228-61-8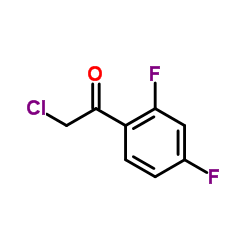 CAS#:51336-94-8
CAS#:51336-94-8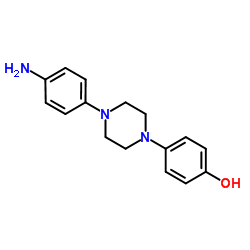 CAS#:74853-08-0
CAS#:74853-08-0
