环已巴比妥
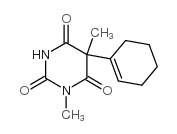
环已巴比妥结构式

|
常用名 | 环已巴比妥 | 英文名 | 2,4,6(1H,3H,5H)-Pyrimidinetrione,5-(1-cyclohexen-1-yl)-1,5-dimethyl- |
|---|---|---|---|---|
| CAS号 | 56-29-1 | 分子量 | 236.26700 | |
| 密度 | 1.225 g/cm3 | 沸点 | 368.8ºC at 760 mmHg | |
| 分子式 | C12H16N2O3 | 熔点 | 145-147° | |
| MSDS | N/A | 闪点 | 176.8ºC | |
| 符号 |



GHS02, GHS06, GHS08 |
信号词 | Danger |
|
Ozonolysis of geraniol-trans, 6-methyl-5-hepten-2-one, and 6-hydroxy-4-methyl-4-hexenal: kinetics and mechanisms.
J. Phys. Chem. A 112(29) , 6636-45, (2008) A combined density functional theory and transition-state theory study of the mechanisms and reaction coefficients of gas-phase ozonolysis of geraniol-trans, 6-methyl-5-hepten-2-one, and 6-hydroxy-4-methyl-4-hexenal is presented. The geometries, energies, and... |
|
|
Rabbit 3-hydroxyhexobarbital dehydrogenase is a NADPH-preferring reductase with broad substrate specificity for ketosteroids, prostaglandin D₂, and other endogenous and xenobiotic carbonyl compounds.
Biochem. Pharmacol. 86(9) , 1366-75, (2013) 3-Hydroxyhexobarbital dehydrogenase (3HBD) catalyzes NAD(P)⁺-linked oxidation of 3-hydroxyhexobarbital into 3-oxohexobarbital. The enzyme has been thought to act as a dehydrogenase for xenobiotic alcohols and some hydroxysteroids, but its physiological functi... |
|
|
Green leaf volatiles enhance methyl jasmonate response in Arabidopsis.
J. Biosci. Bioeng. 114(5) , 540-5, (2012) Plants emit green leaf volatiles (GLVs) in response to insect or pathogen damage. GLVs consist of C6 and C9 aldehydes, alcohols, and their acetate esters, and play important roles in the plant defense response. One of the functions of GLVs in the defense resp... |
|
|
Effects of crop development on the emission of volatiles in leaves of Lycopersicon esculentum and its inhibitory activity to Botrytis cinerea and Fusarium oxysporum.
J. Integr. Plant Biol. 50(1) , 84-91, (2008) Volatiles emitted from the leaves of Lycopersicon esculentum at the two-, ten-leaf and anthesis periods were collected by a gas absorbing method and analyzed by gas chromatography (GC)-mass spectrometry. In total, 33 compounds of volatiles emitted from three ... |
|
|
Effects of Hypericum montbretti extract on the central nervous system and involvement of GABA (A)/Benzodiazepine receptors in its pharmacological activity.
Phytother Res. 26(11) , 1695-700, (2012) The present study was undertaken to investigate the putative activity of a methanol extract of Hypericum montbretti (Guttiferae) on the central nervous system. Rutin (1519 ppm) and quercitrin (784 ppm) were identified as the major phenolic compounds in the ex... |
|
|
Hepatoprotective studies on Sida acuta Burm. f.
J. Ethnopharmacol. 124(2) , 171-5, (2009) Sida acuta Burm. f. (Malvaceae) is used in Indian traditional medicine to treat liver disorders and is useful in treating nervous and urinary diseases and also disorders of the blood and bile.Evaluation of the hepatoprotective properties of the methanolic ext... |
|
|
Sugar beet leaves as new source of hydroperoxide lyase in a bioprocess producing green-note aldehydes.
Biotechnol. Lett. 30(6) , 1115-9, (2008) Hydroperoxide lyase activity was found in sugar beet leaves. Its optimum pH and temperature were, respectively, 6.7 and 22 degrees C. Under these conditions, conversion of linolenic acid 13-hydroperoxide to cis-3-hexenal with a maximum yield of 80% was reache... |
|
|
Hepatoprotective and antioxidant activity of Leucas aspera against D-galactosamine induced liver damage in rats.
Pharm. Biol. 50(12) , 1592-5, (2012) Whole plant of Leucas aspera (LA) Willd. (Labiatae) is traditionally used in Siddha medicine for hepatic ailments.LA was investigated for its hepatoprotective, antioxidant, and protective effect on microsomal drug metabolizing enzymes (MDMEs).LA aqueous extra... |
|
|
Neuropharmacological screening of two 1,5-benzodiazepine compounds in mice.
C R Biol. 333(3) , 214-9, (2010) This work investigates whether the two 1,5-benzodiazepine compounds: 4-(2-hydroxyphenyl)-1,5-benzodiazepin-2-one (RG0501) and Benzopyrano [4,3-c] 1,5-benzodiazepine (RG0502) have any neuropharmacological activities. Diazepam and Flunitrazepam were used as dru... |
|
|
Polymorphism in stereoselective hydroxylations of mephenytoin and hexobarbital by Japanese liver samples in relation to cytochrome P-450 human-2 (IIC9).
Xenobiotica 22(9-10) , 1083-92, (1992) 1. Stereoselective 4'-hydroxylations of R-(--)-mephenytoin and S-(+)-mephenytoin were determined in liver microsomes of 19 Japanese subjects. 2. The content of P-450 human-2 assessed by Western-blots correlated with microsomal S-(+)-mephenytoin 4'-hydroxylati... |