苄叉丙酮
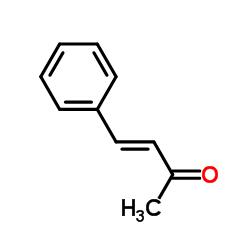
苄叉丙酮结构式

|
常用名 | 苄叉丙酮 | 英文名 | Benzalacetone |
|---|---|---|---|---|
| CAS号 | 122-57-6 | 分子量 | 146.186 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 260.8±9.0 °C at 760 mmHg | |
| 分子式 | C10H10O | 熔点 | 39-42 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 65.6±0.0 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Multiple UDP-glucuronosyltransferases in human liver microsomes glucuronidate both R- and S-7-hydroxywarfarin into two metabolites.
Arch. Biochem. Biophys. 564 , 244-53, (2014) The widely used anticoagulant Coumadin (R/S-warfarin) undergoes oxidation by cytochromes P450 into hydroxywarfarins that subsequently become conjugated for excretion in urine. Hydroxywarfarins may modulate warfarin metabolism transcriptionally or through dire... |
|
|
Dehydrozingerone, chalcone, and isoeugenol analogues as in vitro anticancer agents.
J. Nat. Prod. 69 , 1445-9, (2006) Twenty-eight compounds related to dehydrozingerone (1), isoeugenol (3), and 2-hydroxychalcone (4) were synthesized and evaluated in vitro against human tumor cell replication. Except for isoeugenol analogues 27-35, most compounds exhibited moderate or strong ... |
|
|
Microbial production of 4-hydroxybenzylidene acetone, the direct precursor of raspberry ketone.
Lett. Appl. Microbiol. 45(1) , 29-35, (2007) To investigate the enzymatic aldol reaction between acetone as a donor and 4-hydroxybenzaldehyde as a receptor to generate 4-(4-hydroxyphenyl)-but-3-ene-2-one or 4-hydroxybenzylidene acetone, the direct precursor of 4-(4-hydroxyphenyl)-butan-2-one or raspberr... |
|
|
Synthesis of the pyridinyl analogues of dibenzylideneacetone (pyr-dba) via an improved Claisen-Schmidt condensation, displaying diverse biological activities as curcumin analogues.
Org. Biomol. Chem. 10(6) , 1239-45, (2012) An efficient and easy procedure to synthesize the pyridinyl analogues of dibenzylideneacetone (pyr-dba) was developed by the condensation of substituted nicotinaldehyde and acetone in the presence of K(2)CO(3) in toluene-EtOH-H(2)O solvent system. Structurall... |
|
|
Quantitative structure-activity relationship modeling of antioxidant activities of hydroxybenzalacetones using quantum chemical, physicochemical and spatial descriptors.
Chem. Biol. Drug Des. 73(5) , 526-36, (2009) We have modeled antioxidant activities of hydroxybenzalacetones against lipid peroxidation induced by t-butyl hydroperoxide (pC1), gamma-irradiation (pC2) and also their 1,1-diphenyl-2-picryl hydrazyl (DPPH) free radical scavenging activity (pC3) using quanti... |
|
|
Synthesis and anti-inflammatory activity of novel (substituted)benzylidene acetone oxime ether derivatives: molecular modeling study.
Eur. J. Med. Chem. 45 , 1403-14, (2010) Herein, we report the design, synthesis, and pharmacological properties of a series of substituted benzylidene acetone oxime ether derivatives from the corresponding oxime derivatives. All the newly synthesized compounds were investigated in vivo for their an... |
|
|
Gas phase retro-Michael reaction resulting from dissociative protonation: fragmentation of protonated warfarin in mass spectrometry.
J. Mass Spectrom. 47(8) , 1059-64, (2012) A mass spectrometric study of protonated warfarin and its derivatives (compounds 1 to 5) has been performed. Losses of a substituted benzylideneacetone and a 4-hydroxycoumarin have been observed as a result of retro-Michael reaction. The added proton is initi... |
|
|
DNA-targeting pyrroloquinoline-linked butenone and chalcones: synthesis and biological evaluation.
Eur. J. Med. Chem. 44 , 2854-61, (2009) A series of conjugates of alpha,beta-unsaturated ketone systems, phenyl-butenone and diaryl-propenones (chalcones), with the tricyclic planar pyrroloquinoline nucleus were synthesised and evaluated for their anticancer properties. The aim was to target DNA by... |
|
|
Structure function analysis of benzalacetone synthase from Rheum palmatum.
Bioorg. Med. Chem. Lett. 17(11) , 3161-6, (2007) Benzalacetone synthase (BAS) is a plant-specific chalcone synthase (CHS) superfamily type III polyketide synthase (PKS) that catalyzes a one-step decarboxylative condensation of 4-coumaroyl-CoA with malonyl-CoA. The diketide forming activity of Rheum palmatum... |
|
|
Quantum chemical- and 3-D-QSAR (CoMFA) studies of benzalacetones and 1,1,1-trifluoro-4-phenyl-3-buten-2-ones.
Bioorg. Med. Chem. Lett. 12(17) , 2281-5, (2002) The inhibitory effect (IC(50)) of the title compounds on UV-induced mutagenesis in Escherichia coli WP2uvrA was analyzed quantitatively by using various quantum chemical descriptors and also by the CoMFA method: both approaches provided results of similar qua... |