1,3-双(3,5-双(三氟甲基)苯基)脲
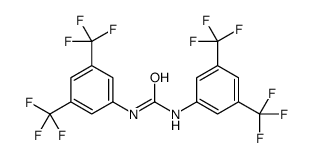
1,3-双(3,5-双(三氟甲基)苯基)脲结构式

|
常用名 | 1,3-双(3,5-双(三氟甲基)苯基)脲 | 英文名 | 1,3-BIS-(ALPHA,ALPHA,ALPHA,ALPHAPR,ALPHAPR,ALPHAPR-HEXAFLUORO-3,5-XYLYL)-UREA |
|---|---|---|---|---|
| CAS号 | 3824-74-6 | 分子量 | 484.23900 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C17H8F12N2O | 熔点 | N/A | |
| MSDS | 美版 | 闪点 | N/A |
|
Urea derivatives are highly active catalysts for the base-mediated generation of terminal epoxides from aldehydes and trimethylsulfonium iodide.
Org. Biomol. Chem. 6(8) , 1339-43, (2008) N,N'-Diarylureas have been shown to efficiently catalyse sulfonium ylide-mediated aldehyde epoxidation reactions for the first time. These processes are of broad scope and can be coupled with a subsequent Cu(II) ion-catalysed Meinwald rearrangement to give an... |
|
|
Internal Lewis acid assisted hydrogen bond donor catalysis.
Org. Lett. 13(4) , 716-9, (2011) Boronate ureas are introduced as a new class of noncovalent catalysts for conjugate addition reactions with enhanced activity. Through intramolecular coordination of the urea functionality to a strategically placed Lewis acid, rate enhancements up to 10 times... |
|
|
Bonding Organocatalysed Friedel-Crafts Alkylation of Aromatic and Heteroaromatic Systems with Nitroolefins Dessole G,et al.
Synlett 13 , 2374-2378, (2004)
|