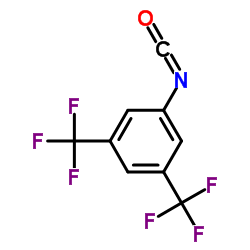
1,3-BIS-(ALPHA,ALPHA,ALPHA,ALPHAPR,ALPHAPR,ALPHAPR-HEXAFLUORO-3,5-XYLYL)-UREA
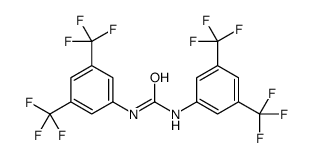
1,3-BIS-(ALPHA,ALPHA,ALPHA,ALPHAPR,ALPHAPR,ALPHAPR-HEXAFLUORO-3,5-XYLYL)-UREA structure
|
Common Name | 1,3-BIS-(ALPHA,ALPHA,ALPHA,ALPHAPR,ALPHAPR,ALPHAPR-HEXAFLUORO-3,5-XYLYL)-UREA | ||
|---|---|---|---|---|
| CAS Number | 3824-74-6 | Molecular Weight | 484.23900 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C17H8F12N2O | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
| Name | 1,3-bis[3,5-bis(trifluoromethyl)phenyl]urea |
|---|---|
| Synonym | More Synonyms |
| Molecular Formula | C17H8F12N2O |
|---|---|
| Molecular Weight | 484.23900 |
| Exact Mass | 484.04500 |
| PSA | 44.62000 |
| LogP | 7.49240 |
| RIDADR | NONH for all modes of transport |
|---|
|
~78% 
1,3-BIS-(ALPHA,... CAS#:3824-74-6 |
| Literature: Denoyelle, Severine; Chen, Ting; Chen, Limo; Wang, Yibo; Klosi, Edvin; Halperin, Jose A.; Aktas, Bertal H.; Chorev, Michael Bioorganic and Medicinal Chemistry Letters, 2012 , vol. 22, # 1 p. 402 - 409 |
|
~69% 
1,3-BIS-(ALPHA,... CAS#:3824-74-6 |
| Literature: Maher, Declan J.; Connon, Stephen J. Tetrahedron Letters, 2004 , vol. 45, # 6 p. 1301 - 1305 |
|
~7% 
1,3-BIS-(ALPHA,... CAS#:3824-74-6 |
| Literature: Busschaert, Nathalie; Kirby, Isabelle L.; Young, Sarah; Coles, Simon J.; Horton, Peter N.; Light, Mark E.; Gale, Philip A. Angewandte Chemie - International Edition, 2012 , vol. 51, # 18 p. 4426 - 4430 |
|
Urea derivatives are highly active catalysts for the base-mediated generation of terminal epoxides from aldehydes and trimethylsulfonium iodide.
Org. Biomol. Chem. 6(8) , 1339-43, (2008) N,N'-Diarylureas have been shown to efficiently catalyse sulfonium ylide-mediated aldehyde epoxidation reactions for the first time. These processes are of broad scope and can be coupled with a subseq... |
|
|
Internal Lewis acid assisted hydrogen bond donor catalysis.
Org. Lett. 13(4) , 716-9, (2011) Boronate ureas are introduced as a new class of noncovalent catalysts for conjugate addition reactions with enhanced activity. Through intramolecular coordination of the urea functionality to a strate... |
|
|
Bonding Organocatalysed Friedel-Crafts Alkylation of Aromatic and Heteroaromatic Systems with Nitroolefins Dessole G,et al.
Synlett 13 , 2374-2378, (2004)
|
| Urea,N,N'-bis[3,5-bis(trifluoromethyl)phenyl] |
| 3,3',5,5'-tetrakis(trifluoromethyl)carbanilide |
| 1,3-bis(3,5-bis(trifluoromethyl)phenyl)urea |
| N,N'-di[3,5-di(trifluoromethyl)phenyl]urea |
| N,N'-Bis-<3,5-bis(trifluormethyl)-phenyl>-harnstoff |
| N-[3,5-Bis(trifluoromethyl)phenyl]{[3,5-bis(trifluoromethyl)phenyl]amino}carboxamide |
| [bis(3,5-trifluoromethyl)benzene]urea |