| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Mattson Boronate Urea Pinacol Ester
CAS:1538637-10-3 |
|
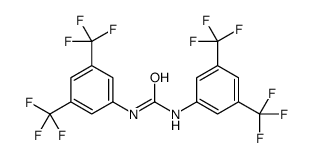 |
1,3-BIS-(ALPHA,ALPHA,ALPHA,ALPHAPR,ALPHAPR,ALPHAPR-HEXAFLUORO-3,5-XYLYL)-UREA
CAS:3824-74-6 |