(-)-没食子酸儿茶素酯;儿茶素没食子酸酯
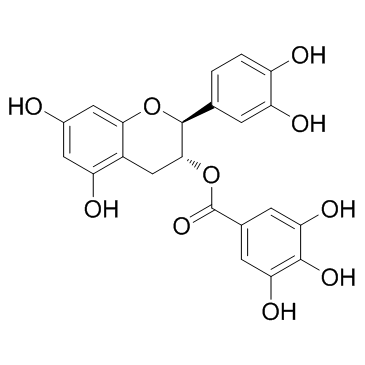
(-)-没食子酸儿茶素酯;儿茶素没食子酸酯结构式

|
常用名 | (-)-没食子酸儿茶素酯;儿茶素没食子酸酯 | 英文名 | (-)-Catechin gallate(CG) |
|---|---|---|---|---|
| CAS号 | 130405-40-2 | 分子量 | 442.372 | |
| 密度 | 1.8±0.1 g/cm3 | 沸点 | 861.7±65.0 °C at 760 mmHg | |
| 分子式 | C22H18O10 | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | 305.0±27.8 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Development and validation of UHPLC-MS/MS method for determination of eight naturally occurring catechin derivatives in various tea samples and the role of matrix effects.
J. Pharm. Biomed. Anal. 114 , 62-70, (2015) A complete analytical procedure combining optimized tea infusion preparation and validated UHPLC-MS/MS method was developed for routine quantification of eight naturally occurring catechin derivatives in various tea samples. The preparation of tea infusions w... |
|
|
Catechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme.
Bioorg. Med. Chem. 16 , 3580-6, (2008) Recent studies have shown that glucose-6-phosphate dehydrogenase (G6PD) is an effectual therapeutic target for metabolic disorders, including obesity and diabetes. In this study, we used in silico and conventional screening approaches to identify putative inh... |
|
|
Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships.
J. Nat. Prod. 70 , 1278-82, (2007) Flavonoids have been recognized as the active ingredients of many medicinal plant extracts due to interactions with proteins via phenolic groups and low toxicity. Here, we report the investigation of the flavonoid core as a potential new scaffold for the deve... |
|
|
Inhibition of Plasmodium falciparum fatty acid biosynthesis: evaluation of FabG, FabZ, and FabI as drug targets for flavonoids.
J. Med. Chem. 49 , 3345-53, (2006) After the discovery of a potent natural flavonoid glucoside as a potent inhibitor of FabI, a large flavonoid library was screened against three important enzymes (i.e., FabG, FabZ, and FabI) involved in the fatty acid biosynthesis of P. falciparum. Although f... |
|
|
Integrated ligand and structure based studies of flavonoids as fatty acid biosynthesis inhibitors of Plasmodium falciparum.
Bioorg. Med. Chem. Lett. 20 , 4779-81, (2010) A common feature pharmacophore with two hydrogen-bond acceptor and one aromatic hydrophobic feature has been generated using seven active flavonoids. Docking studies of these compounds well corroborates with the pharmacophore model. Therefore models could be ... |
|
|
Mass spectrometry-based systems approach for identification of inhibitors of Plasmodium falciparum fatty acid synthase.
Antimicrob. Agents Chemother. 51 , 2552-8, (2007) The emergence of strains of Plasmodium falciparum resistant to the commonly used antimalarials warrants the development of new antimalarial agents. The discovery of type II fatty acid synthase (FAS) in Plasmodium distinct from the FAS in its human host (type ... |