3-叔丁基苯酚
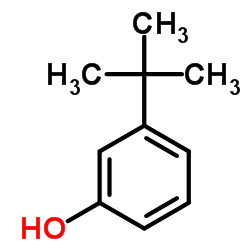
3-叔丁基苯酚结构式

|
常用名 | 3-叔丁基苯酚 | 英文名 | 3-(t-Butyl)phenol |
|---|---|---|---|---|
| CAS号 | 585-34-2 | 分子量 | 150.218 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 240.0±0.0 °C at 760 mmHg | |
| 分子式 | C10H14O | 熔点 | 44-46 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 108.9±0.0 °C | |
| 符号 |

GHS05 |
信号词 | Danger |
|
Convenient QSAR model for predicting the complexation of structurally diverse compounds with β-cyclodextrins
Bioorg. Med. Chem. 17 , 896-904, (2009) This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoretical molecular descriptors, calculated solely from the mole... |
|
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a quantitative structure-activity relationship study.
J. Med. Chem. 48 , 7234-42, (2005) In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop ... |
|
|
Structural features of alkylphenolic chemicals associated with estrogenic activity.
J. Biol. Chem. 272(6) , 3280-8, (1997) The ability of certain man-made chemicals to mimic the effects of natural steroid hormones and their potential to disrupt the delicate balance of the endocrine system in animals are of increasing concern. The growing list of reported hormone-mimics includes t... |
|
|
Isolation and characterization of a novel 2-sec-butylphenol-degrading bacterium Pseudomonas sp. strain MS-1.
Biodegradation 21(2) , 157-65, (2010) A novel bacterium capable of utilizing 2-sec-butylphenol as the sole carbon and energy source, Pseudomonas sp. strain MS-1, was isolated from freshwater sediment. Within 30 h, strain MS-1 completely degraded 1.5 mM 2-sec-butylphenol in basal salt medium, with... |
|
|
Stereoselective hydrogenation of tert-butylphenols over charcoal-supported rhodium catalyst in supercritical carbon dioxide solvent. Hiyoshi N, et al.
J. Catal. 252(1) , 57-68, (2007)
|