1-氟萘
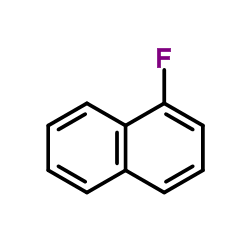
1-氟萘结构式

|
常用名 | 1-氟萘 | 英文名 | 1-Fluoronaphthalene |
|---|---|---|---|---|
| CAS号 | 321-38-0 | 分子量 | 146.161 | |
| 密度 | 1.1±0.1 g/cm3 | 沸点 | 215.0±0.0 °C at 760 mmHg | |
| 分子式 | C10H7F | 熔点 | −13 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 65.6±0.0 °C | |
| 符号 |

GHS08 |
信号词 | Warning |
|
Synthesis of highly oxygenated dinaphthyl ethers via SNAr reactions promoted by Barton's base.
Org. Lett. 5(7) , 1155-8, (2003) [reaction: see text] Electron-rich dinaphthyl ethers were synthesized by S(N)Ar reactions between naphthols and activated fluoronaphthalenes. 2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton's base) was found to be an excellent, mild alternative to tradition... |
|
|
Effects of a fluoro substituent on the fungal metabolism of 1-fluoronaphthalene.
Appl. Environ. Microbiol. 48(2) , 294-300, (1984) The metabolism of 1-fluoronaphthalene by Cunninghamella elegans ATCC 36112 was studied. The metabolites were isolated by reverse-phase high-pressure liquid chromatography and characterized by the application of UV absorption, 1H nuclear magnetic resonance, an... |
|
|
A one-pot access to 6-substituted phenanthridines from fluoroarenes and nitriles via 1,2-arynes.
Org. Lett. 4(16) , 2687-90, (2002) [reaction: see text] A one-pot, t-BuLi-induced synthesis of 6-substituted phenanthridines from fluoroarenes and nitriles via 1,2-arynes is reported. Aryl- and hetaryl nitriles, cyanamides, and trimethylacetonitrile gave phenanthridine products. The method was... |
|
|
Formation of O 2 (1Sigma g+) by 1-fluoronaphthalene sensitization. Andrews LJ and Abrahamson EW.
Chem. Phys. Lett. 10 , 113-6, (1971)
|
|
|
Asymmetric synthesis and absolute stereochemistry of LY248686. Deeter J, et al.
Tetrahedron Lett. 31(49) , 7101-4, (1990)
|