2-氨基苯磺酰胺
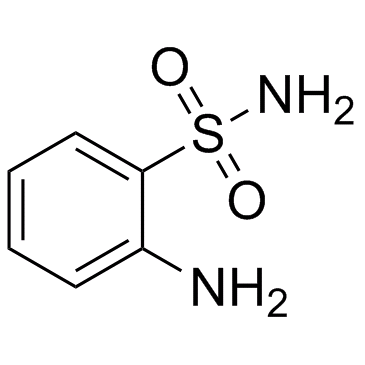
2-氨基苯磺酰胺结构式

|
常用名 | 2-氨基苯磺酰胺 | 英文名 | 2-Aminobenzenesulfonamide |
|---|---|---|---|---|
| CAS号 | 3306-62-5 | 分子量 | 172.205 | |
| 密度 | 1.4±0.1 g/cm3 | 沸点 | 392.7±44.0 °C at 760 mmHg | |
| 分子式 | C6H8N2O2S | 熔点 | 155-157 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 191.3±28.4 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Hepatitis C virus NS3 protease inhibitors comprising a novel aromatic P1 moiety.
Bioorg. Med. Chem. 16(6) , 2955-67, (2008) Inhibition of the hepatitis C virus (HCV) NS3 protease has emerged as an attractive approach to defeat the global hepatitis C epidemic. In this work, we present the synthesis and biochemical evaluation of HCV NS3 protease inhibitors comprising a non-natural a... |
|
|
Nitrogen-15 nuclear magnetic resonance study of benzenesulfonamide and cyanate binding to carbonic anhydrase.
Biochemistry 22 , 2658, (1983)
|
|
|
Heterocycles with a benzothiadiazepine moiety. 5. Derivatives of pyrrolo[2,1-d][1,2,5]benzothiadiazepine, a novel tricyclic ring.
Il Farmaco 52(6-7) , 375-8, (1997) The synthesis of pyrrolo[2,1-d][1,2,5]benzothiadiazepin-7(6H)-one 5,5-dioxide has been achieved by reaction between 2-(1H-pyrrol-1-yl)benzenesulfonamide and triphosgene. N-Ethylation of the tricyclic derivative afforded 6-ethylpyrrolo[2,1-d][1,2,5] benzothiad... |