N,N-二甲基亚甲基碘化铵
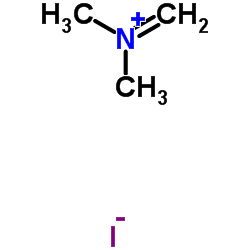
N,N-二甲基亚甲基碘化铵结构式

|
常用名 | N,N-二甲基亚甲基碘化铵 | 英文名 | Eschenmoser's reagent |
|---|---|---|---|---|
| CAS号 | 33797-51-2 | 分子量 | 185.007 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C3H8IN | 熔点 | 219 °C (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | N/A | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Total synthesis and bioactivity of an unnatural enantiomer of merrilactone a: development of an enantioselective desymmetrization strategy.
J. Org. Chem. 72 , 3065, (2007) (-)-Merrilactone A [(-)-1], isolated from Illicium merrillianum in 2000, possesses neurite outgrowth activity in cultures of fetal rat cortical neurons, and, therefore, is expected to show therapeutic potential for the treatment of neurodegeneration associate... |
|
|
J. Prakt. Chem./Chem.-Ztg. 336 , 91, (1994)
|
|
|
Chem. Abstr. 120 , 190555b, (1994)
|
|
|
Tetrahedron Asymmetry 5 , 921, (1994)
|
|
|
J. Chem. Soc. Perkin Trans. I , 3295, (1992)
|
|
|
A new method using 2-chloro-4, 6-dimethoxy-1, 3, 5-triazine for facile elimination of dimethylamino group in Eschenmoser's methylenation for synthesis of a, ß-unsaturated esters. Yamada K, et al.
Tetrahedron Lett. 54(13) , 1753-60, (2013)
|
|
|
Synthetic Routes to Unsymmetrical Porphyrins. Nardis S.
Synthesis and Modifications of Porphyrinoids , (2014), 203-39
|