5-氯吲哚-3-甲醛
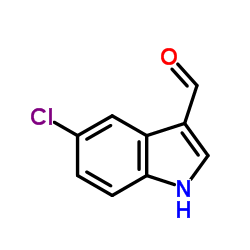
5-氯吲哚-3-甲醛结构式

|
常用名 | 5-氯吲哚-3-甲醛 | 英文名 | 5-Chloroindole-3-carboxaldehyde |
|---|---|---|---|---|
| CAS号 | 827-01-0 | 分子量 | 179.603 | |
| 密度 | 1.4±0.1 g/cm3 | 沸点 | 373.4±22.0 °C at 760 mmHg | |
| 分子式 | C9H6ClNO | 熔点 | 213-216 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 179.6±22.3 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Synthesis and antifungal activity of novel streptochlorin analogues.
Eur. J. Med. Chem. 92 , 776-83, (2015) Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of pimprinine... |
|
|
Electrochemical behavior of indole-3-carboxaldehyde izonicotinoyl hydrazones: discussion on possible biological behavior Shirinzadeh H, et al.
Comb. Chem. High Throughput Screen 13(7) , 619-627, (2010)
|
|
|
2′-[(5-Chloro-1H-indol-3-yl) methylene]-2-(1H-indol-3-yl) acetohydrazide. Ali HM, et al.
Acta Crystallogr. Sect. E Struct. Rep. Online 63(4) , o1807-o1808, (2007)
|
|
|
Tandem Suzuki-Miyaura cross-coupling/dehydrobromination of 1, 1-dibromoalkenes to alkynes with a cyclobutene-1, 2-diylbis (imidazolium) salt as catalyst precursor. Rahimi A and Schmidt A.
Synthesis 2010(15) , 2621-2625, (2010)
|
期刊文献综述:
更多...