己酰胺
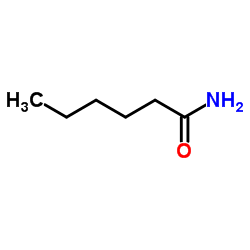
己酰胺结构式

|
常用名 | 己酰胺 | 英文名 | n-Hexanamide |
|---|---|---|---|---|
| CAS号 | 628-02-4 | 分子量 | 115.174 | |
| 密度 | 0.9±0.1 g/cm3 | 沸点 | 255.0±0.0 °C at 760 mmHg | |
| 分子式 | C6H13NO | 熔点 | 100-102 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 102.2±18.4 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Zirconium-mediated conversion of amides to nitriles: a surprising additive effect.
Angew. Chem. Int. Ed. Engl. 43(40) , 5375-7, (2004)
|
|
|
Aliphatic and enantioselective amidases: from hydrolysis to acyl transfer activity.
J. Appl. Microbiol. 91(3) , 381-93, (2001)
|
|
|
Carcinogenesis bioassay of acetamide, hexanamide, adipamide, urea and P-tolylurea in mice and rats.
J. Environ. Pathol. Toxicol. 3(5-6) , 149-70, (1980) As a part of the National Cancer Institute's effort to screen environmental and occupational chemicals for chronic toxicity and carcinogenicity, the amides acetamide, hexanamide, adipamide, urea, and p-tolylurea were fed to male and female C57B1/6 mice and Fi... |
|
|
Development of a thyroid hormone receptor targeting conjugate.
Bioconjug. Chem. 19(6) , 1227-34, (2008) Molecular conjugates of hormone receptor-ligands with molecular probes or functional domains are finding diverse applications in chemical biology. Whereas many examples of hormone conjugates that target steroid hormone receptors have been reported, practical ... |
|
|
N-(3-Triethoxysilylpropyl)-6-(N-maleimido)-hexanamide: An efficient heterobifunctional reagent for the construction of oligonucleotide microarrays.
Anal. Biochem. 357(2) , 240-8, (2006) Synthesis of a new heterobifunctional reagent, N-(3-triethoxysilylpropyl)-6-(N-maleimido)-hexanamide (TPMH), for the preparation of oligonucleotide microarrays is described. Its triethoxysilyl function is specific toward virgin glass surface and maleimide fun... |
|
|
Preparation and characterization of galactose-modified liposomes by a nonaqueous enzymatic reaction.
J. Liposome Res. 21(3) , 255-60, (2011) In this study, NOH (NOH = N-octadecyl-4-[(D-galactopyranosyl)oxy]-2,3,5,6-tetrahydroxy hexanamide) was enzymatically synthesized as a targeting molecule and incorporated into liposomes to prepare a liposome surface modified with galactose. Glycyrrhetinic-acid... |