3-氨基苯硼酸 一水合物
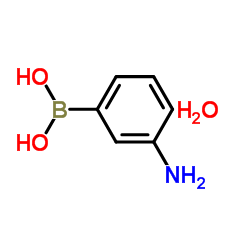
3-氨基苯硼酸 一水合物结构式

|
常用名 | 3-氨基苯硼酸 一水合物 | 英文名 | 3-Aminophenylboronic acid monohydrate |
|---|---|---|---|---|
| CAS号 | 206658-89-1 | 分子量 | 154.96 | |
| 密度 | 1.23 g/cm3 | 沸点 | 376ºC at 760 mmHg | |
| 分子式 | C6H10BNO3 | 熔点 | 94 °C | |
| MSDS | 中文版 美版 | 闪点 | 181.2ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Development of quantum dots-based biosensor towards on-farm detection of subclinical ketosis.
Biosens. Bioelectron. 72 , 140-7, (2015) Early detection of dairy animal health issues allows the producer or veterinarian to intervene before the animals' production levels, or even survival, is threatened. An increased concentration of β-hydroxybutyrate (βHBA) is a key biomarker for diagnosis of s... |
|
|
Sugar-boronate ester scaffold tethered pyridyl-imine palladium(II) complexes: synthesis and their in vitro anticancer evaluation.
Dalton Trans. 44(40) , 17600-16, (2015) A series of five palladium(ii) pyridyl-imine Schiff base complexes 5a-e containing boronate esters with protected sugar diols derived from d-xylose, l-sorbose and d-mannitol were designed and synthesized starting from pyridyl-imines generated in situ from 3-a... |
|
|
Synthetic studies connected with the preparation of N-[3-(3-cyanopyrazolo[1,5-a]pyrimidin-5-yl)phenyl]-N-ethylacetamide, a zaleplon regioisomer Radl, S.; et al.
Heterocycles 2nd ed., 80 , 1359-1379, (2010)
|
|
|
Amphiphilic random glycopolymer based on phenylboronic acid: synthesis, characterization, and potential as glucose-sensitive matrix.
Biomacromolecules 6th ed., 10 , 1337-1345, (2009) This study is devoted to developing amphiphilic, random glycopolymers based on phenylboronic acid, which self-assemble to form nanoparticles (NPs), as a glucose-sensitive agent. Maleimide-glucosamine was copolymerized with 3-acryl aminophenylboronic acid in m... |
|
|
A chemomechanical polymer that functions in blood plasma with high glucose selectivity.
Angew. Chem. Int. Ed. Engl. 32th ed., 45 , 5319-5322, (2006)
|
|
|
Towards Gram-positive antivirulence drugs: new inhibitors of Streptococcus agalactiae Stk1.
Bioorg. Med. Chem. Lett. 12th ed., 20 , 3486-3490, (2010) A structure-activity relationship study from a screening hit and structure-based design strategy has led to the identification of bisarylureas as potent inhibitors of Streptococcus agalactiae Stk1. As this target has been directly linked to bacterial virulenc... |