4-甲基伞形酮基α-D-吡喃甘露糖苷
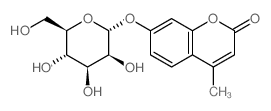
4-甲基伞形酮基α-D-吡喃甘露糖苷结构式

|
常用名 | 4-甲基伞形酮基α-D-吡喃甘露糖苷 | 英文名 | 4-methylumbelliferyl beta-D-mannopyranoside |
|---|---|---|---|---|
| CAS号 | 28541-83-5 | 分子量 | 338.30900 | |
| 密度 | 1.522 g/cm3 | 沸点 | 626.9ºC at 760 mmHg | |
| 分子式 | C16H18O8 | 熔点 | 190ºC | |
| MSDS | 美版 | 闪点 | 233.9ºC |
|
Binding of manno-oligosaccharides to concanavalin A. Substitution titration with a fluorescent-indicator ligand.
Eur. J. Biochem. 103(2) , 307-12, (1980) The association constants for binding of methyl alpha-D-mannopyranoside (I), mannobiose (II) and mannotriose (III) to concanavalin A were determined in the temperature range 285-313 K by a substitution titration, using 4-methylumbelliferyl alpha-D-mannopyrano... |
|
|
Enzyme studies on human and snail beta-mannosidase using a fluorescence assay and an HPLC/diode array method with Man beta (1-4)GlcNAc as substrate.
Int. J. Biochem. 24(4) , 669-73, (1992) 1. Snail beta-mannosidase showed a Km value of 0.05 mM toward MU-beta-Man and could not be inhibited by Man, GlcNAc, Man beta(1-4)GlcNAc, Man beta(1-4)GlcNAc beta(1-N)urea or Man beta(1-4) GlcNAc beta(1-4)GlcNAc. 2. The Km value of the snail enzyme towards Ma... |
|
|
Kinetics of interaction of some alpha- and beta-D-monosaccharides with concanavalin A.
Biochim. Biophys. Acta 631(3) , 428-38, (1980) The rates of formation and dissociation of concanavalin A with some 4-methylumbelliferyl and p-nitrophenyl derivatives of alpha- and beta-D-mannopyranosides and glucopyranosides were measured by fluorescence and spectral stopped-flow methods. All processes ex... |
|
|
The putative role of members of the CEA-gene family (CEA, NCA an BGP) as ligands for the bacterial colonization of different human epithelial tissues.
Zentralbl. Bakteriol. 275(1) , 118-22, (1991) Immobilized purified CEA (carcinoembryonic antigen), NCA (non-specific crossreacting antigen) and BGP I (biliary glycoprotein I) bind strains of E. coli (including EPEC) and some Salmonella species (including S. typhi, S. paratyphi A + B and S. java) while Sh... |
|
|
Binding of methylumbelliferyl mannoside to concanavalin A under high pressure.
Biochim. Biophys. Acta 790(1) , 87-90, (1984) The equilibrium binding of 4-methylumbelliferyl alpha-D-mannopyranoside to concanavalin A was measured by changes in fluorescence quenching observed at pressures ranging from 1 to 2000 bar (1974 atmospheres). From the pressure-induced changes in the apparent ... |
|
|
The soluble alpha-mannosidases of Drosophila melanogaster.
Insect Biochem. Mol. Biol. 27(7) , 657-61, (1997) The alpha-mannosidases are implicated in both the catabolism of carbohydrates and the N-linked glycosylation pathway in insects, but little is known of the biochemistry of these glycosidases. In order to study the soluble alpha-mannosidases of Drosophila mela... |
|
|
Binding kinetics of methyl alpha-D-mannopyranoside to concanavalin A: temperature-jump relaxation study with 4-methylumbelliferyl alpha-D-mannopyranoside as a fluorescence indicator ligand.
Biochemistry 20(16) , 4687-92, (1981) The binding of methyl alpha-D-mannopyranoside and methyl alpha-D-glucopyranoside to concanavalin A has been investigated by the temperature-jump relaxation kinetic technique using the competitive inhibitor 4-methylumbelliferyl alpha-D-mannopyranoside as an in... |
|
|
Manganese, calcium, and saccharide binding to concanavalin A, as studied by ultrafiltration.
Arch. Biochem. Biophys. 223(2) , 350-9, (1983) The binding of the ligands Mn2+, Ca2+, and methyl alpha-D-glucopyranoside to concanavalin A, purified as described (A.J. Sophianopoulos and J.A. Sophianopoulos (1981) Prep. Biochem. 11, 413-435), was studied by ultrafiltration in 0.2 M NaCl, pH 5.2 and pH 6.5... |
|
|
Activation of concanavalin A by Cd2+.
J. Biol. Chem. 255(18) , 8772-5, (1980) Binding of Cd2+ to concanavalin A and the subsequent induction of saccharide-binding activity has been studied at pH 6.5. We found that Cd2+ bound to both metal sites, S1 and S2, and that Cd2+ alone would induce sugar binding in concanavalin A. Using the fluo... |
|
|
Class II α-mannosidase fromAspergillus fischeri: Energetics of catalysis and inhibition
Int. J. Biol. Macromol. 44(1) , 112-5, (2009) Energetics of the catalysis of Class II α-mannosidase (E.C.3.2.1.24) from Aspergillus fischeri was studied. The enzyme showed K cat/ K m for Man (α1-3) Man, Man (α1-2) Man and Man (α1-6) Man as 7488, 5376 and 3690 M −1 min −1, respectively. The activation ene... |