Kinetics of interaction of some alpha- and beta-D-monosaccharides with concanavalin A.
R D Farina, R G Wilkins
文献索引:Biochim. Biophys. Acta 631(3) , 428-38, (1980)
全文:HTML全文
摘要
The rates of formation and dissociation of concanavalin A with some 4-methylumbelliferyl and p-nitrophenyl derivatives of alpha- and beta-D-mannopyranosides and glucopyranosides were measured by fluorescence and spectral stopped-flow methods. All processes examined were uniphasic. The second-order formation rate constants varied only from 6.8 x 10(4) to 12.8 x 10(4) M-1 x s-1, whereas the first-order dissociation rate constants ranged from 4.1 to 220 s-1, all at pH 5.0, I=0.3 M, and 25 degrees C. Dissociation rates thus controlled the value of the binding constant. The effect of temperature on these reactions was examined, from which enthalpies and entropies of activation and of reaction could be calculated. The effects of pH at 25 degrees C on the reaction rates of 4-methylumbelliferyl alpha-D-mannopyranoside and 4-methylumbelliferyl alpha-D-glucopyranoside with concanavalin A were examined. The value of the binding constant Kap (derived from the kinetics) at any pH could be related to the intrinsic binding constant K by the expression Kap = KaK(Ka + [H+])-1. The values of Ka, the ionization constant of the protein segment responsive to sugar binding, were 3 x 10(-4) M and 1 x 10(-4) M for 4-methylumbelliferyl alpha-D-mannopyranoside and 4-methylumbelliferyl alpha-D-glucopyranoside, respectively. The binding constant of p-nitrophenyl alpha-D-mannopyranoside is surprisingly much less sensitive to a pH change from 5.0 to 2.7. Ionic strength had little effect on the binding characteristics of 4-methylumbelliferyl alpha-D-mannopyranoside to concanavalin A at pH 5.2 and 25 degrees C.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
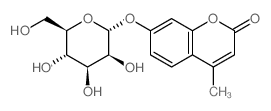 |
4-甲基伞形酮基α-D-吡喃甘露糖苷
CAS:28541-83-5 |
C16H18O8 |
|
Binding of manno-oligosaccharides to concanavalin A. Substit...
1980-01-01 [Eur. J. Biochem. 103(2) , 307-12, (1980)] |
|
Enzyme studies on human and snail beta-mannosidase using a f...
1992-04-01 [Int. J. Biochem. 24(4) , 669-73, (1992)] |
|
The putative role of members of the CEA-gene family (CEA, NC...
1991-04-01 [Zentralbl. Bakteriol. 275(1) , 118-22, (1991)] |
|
Binding of methylumbelliferyl mannoside to concanavalin A un...
1984-10-09 [Biochim. Biophys. Acta 790(1) , 87-90, (1984)] |
|
The soluble alpha-mannosidases of Drosophila melanogaster.
1997-07-01 [Insect Biochem. Mol. Biol. 27(7) , 657-61, (1997)] |