华蟾毒精
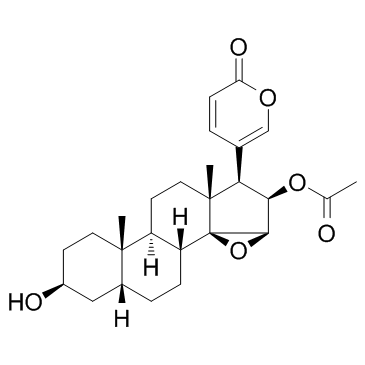
华蟾毒精结构式

|
常用名 | 华蟾毒精 | 英文名 | Cinobufagin |
|---|---|---|---|---|
| CAS号 | 470-37-1 | 分子量 | 442.545 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 595.4±50.0 °C at 760 mmHg | |
| 分子式 | C26H34O6 | 熔点 | 222-223ºC | |
| MSDS | 中文版 美版 | 闪点 | 199.4±23.6 °C | |
| 符号 |

GHS06 |
信号词 | Danger |
|
Microbial transformation of cinobufagin by Syncephalastrum racemosum.
J. Nat. Prod. 71 , 1268-70, (2008) Microbial transformation of a cytotoxic bufadienolide, cinobufagin (1), was performed by Syncephalastrum racemosum. The six metabolites obtained were identified as 7beta-hydroxycinobufagin ( 2), 12beta-hydroxycinobufagin (3), cinobufotalin (4), 5,12beta-dihyd... |
|
|
Preparative separation of four major bufadienolides from the Chinese traditional medicine, Chansu, using high-speed counter-current chromatography.
Nat. Prod. Commun. 5(7) , 1031-4, (2010) A preparative, high-speed, counter-current chromatographic (HSCCC) method for the isolation and purification of bufadienolides from Chansu was successfully developed by using stepwise elution with a two-phase solvent system composed of n-hexane: chloroform: m... |
|
|
Cinobufacini induced MDA-MB-231 cell apoptosis-associated cell cycle arrest and cytoskeleton function.
Bioorg. Med. Chem. Lett. 22(3) , 1459-63, (2012) Cinobufacini is a traditional Chinese anti-tumor drug and widely used in clinic experiences. But little is known about its effect on the cells. In this study, the effects of cinobufacini on breast cancer MDA-MB-231 cell were evaluated by CCK-8 assay, and the ... |
|
|
Bufothionine, a possible effective component in cinobufocini injection for hepatocellular carcinoma
J. Ethnopharmacol. 141(2) , 692-700, (2012) Structures of bufothionine (A), buffalin (B), cinobufagin (C) and resibufogenin (D). |
|
|
Qualitative and quantitative analysis of cinobufacini injection using rapid separation liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry and HPLC-photodiode array detection, a feasible strategy for the quality control of Chinese medicine injections.
J. Sep. Sci. 36(3) , 492-502, (2013) Cinobufacini injection, prepared from the skin of Bufo bufo gargarizans Cantor, has presented its significant effects on the treatment of hepatitis and various cancers in the clinic. However, as an unclear complex chemical system, the optimization of its qual... |
|
|
Identification of cinobufagin metabolites in the bile of rats.
Xenobiotica 40(1) , 48-54, (2010) Cinobufagin (1) is a major bufadienolide in ChanSu (a traditional Chinese medicine) with a wide range of pharmacological activities. In this paper, the in vivo metabolites of 1 in rats were studied. Nine metabolites were isolated from the bile of rats, and th... |
|
|
Inhibitory effect of bufalin and cinobufagin on steroidogenesis via the activation of ERK in human adrenocortical cells.
Br. J. Pharmacol. 165(6) , 1868-76, (2012) Bufalin and cinobufagin exhibit cardiotonic and natriuretic activities. The aim of this study was to evaluate the effects of bufalin and cinobufagin on aldosterone and cortisol secretion and their mechanisms of action in human adrenocortical cells (NCI-H295).... |
|
|
Microbial transformation of three bufadienolides by Nocardia sp. and some insight for the cytotoxic structure-activity relationship (SAR).
Bioorg. Med. Chem. Lett. 17 , 6062-5, (2007) Resibufogenin, cinobufagin, and bufalin are cytotoxic steroids isolated from the Chinese drug Chan'su. Biotransformation of these three bufadienolides by Nocardia sp. NRRL 5646 was investigated. Notably, resibufogenin was converted to 3-acetyl 15beta-hydroxyl... |
|
|
Effects of Resibufogenin and Cinobufagin on voltage-gated potassium channels in primary cultures of rat hippocampal neurons
Toxicol. In Vitro 25(8) , 1644-53, (2011) Highlights ► Resibufogenin inhibited both IK and IA in cultured rat hippocampal neurons. ► Cinobufagin inhibited IK without noticeable effect on IA in hippocampal neurons. ► Resibufogenin and Cinobufagin altered some channel kinetics of IK. ► Resibufogenin an... |
|
|
Efficient isolation and purification of five products from microbial biotransformation of cinobufagin by high-speed counter-current chromatography.
J. Sep. Sci. 33(15) , 2272-7, (2010) An efficient separation method of using high-speed counter-current chromatography was successfully established to directly purify cytotoxic transformed products of cinobufagin by Cordyceps militaris. The two-phase solvent system composed of n-hexane-ethyl ace... |