7-溴庚酸乙酯
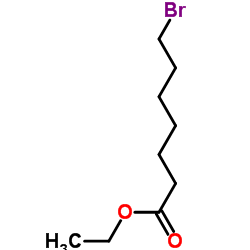
7-溴庚酸乙酯结构式

|
常用名 | 7-溴庚酸乙酯 | 英文名 | Ethyl 7-bromoheptanoate |
|---|---|---|---|---|
| CAS号 | 29823-18-5 | 分子量 | 237.13 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 248.5±23.0 °C at 760 mmHg | |
| 分子式 | C9H17BrO2 | 熔点 | 29 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 129.0±13.0 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Iron oxide nanoparticles functionalized with novel hydrophobic and hydrophilic porphyrins as potential agents for photodynamic therapy.
J. Colloid. Interface Sci. 462 , 154-65, (2015) The preparation of novel porphyrin derivatives and their immobilization onto iron oxide nanoparticles to build up suitable nanotools for potential use in photodynamic therapy (PDT) has been explored. To achieve this purpose, a zinc porphyrin derivative, ZnPR-... |
|
|
Oxime amides as a novel zinc binding group in histone deacetylase inhibitors: synthesis, biological activity, and computational evaluation.
J. Med. Chem. 54(7) , 2165-2182, (2011) Several oxime containing molecules, characterized by a SAHA-like structure, were explored to select a potentially new biasing binding element for the zinc in HDAC catalytic site. All compounds were evaluated for their in vitro inhibitory activity against the ... |
|
|
Nitrones for understanding and ameliorating the oxidative stress associated with aging.
Age (Dordr.) 31(4) , 269-76, (2009) Oxidative damage from reactive oxygen species (ROS) and the carbon-centred radicals arising from them is important to the process of aging, and age-related diseases are generally caused, exacerbated or mediated by oxidative stress. Nitrones can act as spin tr... |
|
|
Design Principle of Conjugated Polyelectrolytes to Make Them Water-Soluble and Highly Emissive. Lee K, et al.
Adv. Funct. Mater. 22(5) , 1076-1086, (2012)
|
|
|
Synthesis of a Prostaglandin Intermediate and Synthesis of Dihydrojasmone and Methyl Dihydrojasmonate. Naoshima Y, et al.
Agric. Biol. Chem. 43(8) , 1765-1768, (1979)
|
|
|
David J Hart
Organic Synthesis via Examination of Selected Natural Products , 99
|
|
|
Synthesis of ω-chain shortened prostaglandins and their six-membered ring analogs. Naoshima Y, et al.
Agric. Biol. Chem. 48(3) , 783-787, (1984)
|