lithium-7
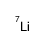
lithium-7结构式

|
常用名 | lithium-7 | 英文名 | lithium-7 |
|---|---|---|---|---|
| CAS号 | 13982-05-3 | 分子量 | 7.01600 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | Li | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | N/A | |
| 符号 |


GHS02, GHS05 |
信号词 | Danger |
|
Optimized Treatment of Heparinized Blood Fractions to Make Them Suitable for Analysis.
Biopreserv. Biobank. 13 , 287-95, (2015) It has been known for decades that many cytokines, such as IL-2, IL-6, and IL-12, bind to heparin. Even though some enzyme-linked immunosorbent assays (ELISA) use antibody-recognizing epitopes not affected by this binding, ELISA manufacturers often warn that ... |
|
|
DMSO-Li2O2 Interface in the Rechargeable Li-O2 Battery Cathode: Theoretical and Experimental Perspectives on Stability.
ACS Appl. Mater. Interfaces 7 , 11402-11, (2015) One of the greatest obstacles for the realization of the nonaqueous Li-O2 battery is finding a solvent that is chemically and electrochemically stable under cell operating conditions. Dimethyl sulfoxide (DMSO) is an attractive candidate for rechargeable Li-O2... |
|
|
Tissue-like Silicon Nanowires-Based Three-Dimensional Anodes for High-Capacity Lithium Ion Batteries.
Nano Lett. 15 , 3907-16, (2015) Here, we report on the scalable synthesis and characterization of novel architecture three-dimensional (3D) high-capacity amorphous silicon nanowires (SiNWs)-based anodes with focus on studying their electrochemical degradation mechanisms. We achieved an unpr... |
|
|
Mechanism and Kinetics of Li2S Precipitation in Lithium-Sulfur Batteries.
Adv. Mater. 27 , 5203-9, (2015) The kinetics of Li2 S electrodeposition onto carbon in lithium-sulfur batteries are characterized. Electrodeposition is found to be dominated by a 2D nucleation and growth process with rate constants that depend strongly on the electrolyte solvent. Nucleation... |
|
|
MnCo2O4 nanowires anchored on reduced graphene oxide sheets as effective bifunctional catalysts for Li-O2 battery cathodes.
ChemSusChem 8 , 1752-60, (2015) A hybrid composite system of MnCo2 O4 nanowires (MCO NWs) anchored on reduced graphene oxide (RGO) nanosheets was prepared as the bifunctional catalyst of a Li-O2 battery cathode. The catalysts can be obtained from the hybridization of one-dimensional MCO NWs... |
|
|
In Situ-Grown ZnCo2O4 on Single-Walled Carbon Nanotubes as Air Electrode Materials for Rechargeable Lithium-Oxygen Batteries.
ChemSusChem 8 , 3697-703, (2015) The development of highly efficient catalysts is critical for the practical application of lithium-oxygen (Li-O2) batteries. Nanosheet-assembled ZnCo2O4 (ZCO) microspheres and thin films grown in situ on single-walled carbon nanotube (ZCO/SWCNT) composites as... |
|
|
A Polymer Lithium-Oxygen Battery.
Sci. Rep. 5 , 12307, (2015) Herein we report the characteristics of a lithium-oxygen battery using a solid polymer membrane as the electrolyte separator. The polymer electrolyte, fully characterized in terms of electrochemical properties, shows suitable conductivity at room temperature ... |
|
|
Single step transformation of sulphur to Li2S2/Li2S in Li-S batteries.
Sci. Rep. 5 , 12146, (2015) Lithium-sulphur batteries have generated tremendous research interest due to their high theoretical energy density and potential cost-effectiveness. The commercial realization of Li-S batteries is still hampered by reduced cycle life associated with the forma... |
|
|
Use of graphite as a highly reversible electrode with superior cycle life for sodium-ion batteries by making use of co-intercalation phenomena.
Angew. Chem. Int. Ed. Engl. 53(38) , 10169-73, (2014) Although being the standard anode material in lithium-ion batteries (LIBs), graphite so far is considered to fail application in sodium-ion batteries (NIBs) because the Na-C system lacks suitable binary intercalation compounds. Here we show that this limitati... |
|
|
Mass-transport Control on the Discharge Mechanism in Li-O2 Batteries Using Carbon Cathodes with Varied Porosity.
ChemSusChem 8 , 3465-71, (2015) By comparing carbon electrodes with varying porosity in Li-O2 cells, we show that the effect of electrolyte stirring at a given current density can result in a change from 2D to 3D growth of discharged deposits. The change of morphology is evident using elect... |